Home » Chelating Resin Manufacturer
Home » Chelating Resin Manufacturer
Chelating resins have always been the best technology to remove heavy metals, transition cations, and metal recovery applications. These resins are highlighted from other typical resins due to their unique selectivity of targeted ions in an environment of a high concentration of competitive ions.
Felite™ Resin Technology introduces a wide range of chelating resins with a high affinity to heavy metal ions adsorption in hydrometallurgy applications due to the attached special functional groups.
Contact the Felite™ team for the best consultation to find the best resin for your application.
Natural water resources are contaminated with heavy metal effluents disposed of in many industries in the fast-moving world. Heavy metal effluent disposal severely damages the natural ecosystem; even humans can be affected by them. Hence, there are rules and regulations for permissible levels of heavy metal effluent disposal in public water sources.

The water purification industry introduces various technologies for heavy metal removal; chemical precipitation, ion exchange, membrane filtration, and carbon adsorption. Among these conventional processes, ion exchange and adsorption are the effective and efficient technologies that are the most popular worldwide.
On many occasions, ion exchange processes offer design and operational flexibility with colorless, odorless, reusable treated water. Also, the ion exchange method is economically supported while regenerating adsorbents in the reversible process.
Modern ion exchange technology uses specially modified resin types introduced by resin manufacturers. The resins were prepared with styrene crosslinked with divinyl benzene (DVB), polyacrylate, melamine formaldehyde diethylenetriaminepentaacetic acid (MF-DTPA), and poly-glycidyl methacrylate-iminodiacetic acid [poly (GMA-IDA)], etc. in common. It is identified that IDA is an excellent functional group in chelating resins which expresses good absorption capacity for some common toxic heavy metals.
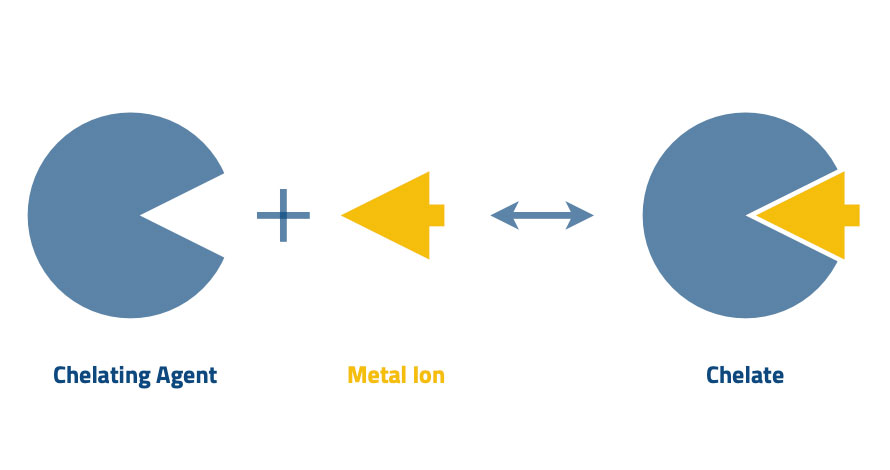
Chelating resins can be categorized under the sub-group of ion exchange resins. They are specialized in removing and recovering heavy metal ions in industrial waste solutions due to high selectivity for targeted metal cations. All the chelating resins exchange cations with respect to the chelation properties. The ligands (chelating resins’ functional group) do not exchange anions and anion complexes due to the formation of various complexes between many metals and chemicals. It needs to focus on feed water ion composition rather than the interesting metal ions.
The ion exchange process generally has a dynamic equilibrium between the solid resin and the selected solution type. With the know composition of the liquid, the equilibrium is also predictable. Rapid change of liquid composition causes the rapid equilibrium between the resin and solid. It results in the unloading of metals because chelate resins operate with limited inlet composition of the liquid.
We consider chelating resins are selective for the targeted ions, but it is selective for the ion groups. In some cases, all cation resins have a sense for all cations. For example, imagine there is a resin that is highly selective for copper ions, but in the presence of calcium, that resin isn’t effectively selective for copper. In the same way, that resin is selective for the other divalent metal cations present in the solution. Chelating resin can’t behave in highly acidic environments.
Chelating resin is another type of ion exchange resin with complex bonds (ligands) with metal cations. Further, we can explain the following. The ligands are formed due to stabilizing the charge balance between the resin and the metal. The resin has exposed lone electron pairs, and metal ion search for electrons to stabilize the charge balance. Commercial chelating resins express both chelation and ion exchange functionalities. So they use ion exchange and the chelation processes to capture the metal cations.
The selectivity of the chelating resins can be defined as the inclination of the resin for the desired ion. Selectivity always depends on the operating conditions. Why do we say so? Because chelating resins’ exchange rate can be limited due to the chelant bonds’ stability and the function group of the chelating resin. Therefore, there may have capacity losses and metal leakages. The chelate resins are very selective for the particular ion due to their valance, ligands bonding, and hydrated ion radius.
Chelating resins usually have two parts; polymer matrix and the chelation group. The polymer matrix can be synthetic and natural, which are insoluble in water. Synthetic resins are more common for commercial purposes, and most of them are crosslinked polymers such as styrene and divinylbenzene (DVB). There are natural polymers, but we have not discussed them here. Chelation groups or various ligands with N, O, and S that have donor atoms are copolymerized with a polymer matrix to form chelating resins.
Chelate resins are stable with an entire pH range of 0-14.
The resin industry uses three types of common chelating resin types, and they are; Iminodiacetate chelant, Aminophosphonic chelant, and Picolyamine chelant. Also, there are pyridine chelant and amoxidine chelant that are not used in common. There are more selective resins, except true chelate resins, that can use for metal removal. Some examples are natural zeolite, weak acid cation resins, and weakly basic polyamine resins.
The functional group of the iminodiacetate resins has two carboxylic acid groups bonded to a nitrogen atom, and the entire group is bonded to the resins’ polymer structure. Exchanging theory of carboxylic group is the same as weak acid cation resins. The function of the nitrogen atom is to provide a Lewis base to form ligands with metal cations in the solution. This action gives the resins a high selectivity for metals widely used in the heavy metal removal industry. Ion exchange and the chelation together remove the metal ions. The charge balance of the exchange group is stable with the attachment of hydrogen ions to the carboxylic groups. Therefore, carboxylic groups do not exchange many cations, and resins have limited chelating capabilities.
The operations of iminodiacetate chelating resins can be affected by the dissolved solids. There are no issues with Sodium and Calcium (group 02 elements). The selectivity is affected by other agents; EDTA, Ammonia, and chloride. EDTA can block the exchange of many metals, and chloride has less effect on selectivity. The slightly acidic pH levels are the best condition for selectivity, and increasing pH levels lower the selectivity ability. FS400 selects Cu2+, Pb2+ at minimum pH 2, and Co2+, Mg2+, Ni2+ with minimum pH 4.5.
Felite™ introduces macro-porous weakly based chelating anion resins for Boron (B) removal from brine, ultra-pure water, and portable water. FS700 has the chemical structure of chemically bounded N-methylglucamine functional group to the polystyrene crosslinked with divinylbenzene (DVB) that has impressive stability in strong osmotic pressure and high thermal shocks. It shows high selectivity for the borate ions in solutions.
The functional group of N-methylglucamine can be shown as follows.
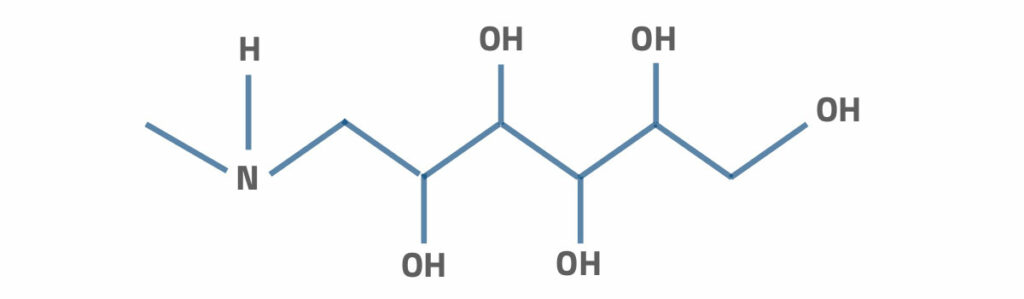
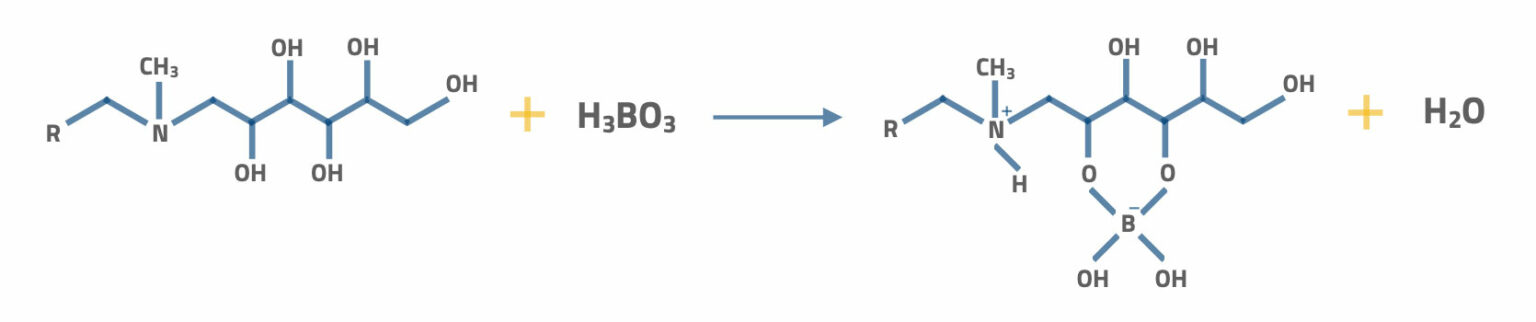
This N-methylglucamine can also express as N-methyl (polyhydroxohexyl) amine. The selectivity is very high for Boron in the form of trioxyboric acid. The following process shows the Boron sorption clearly.
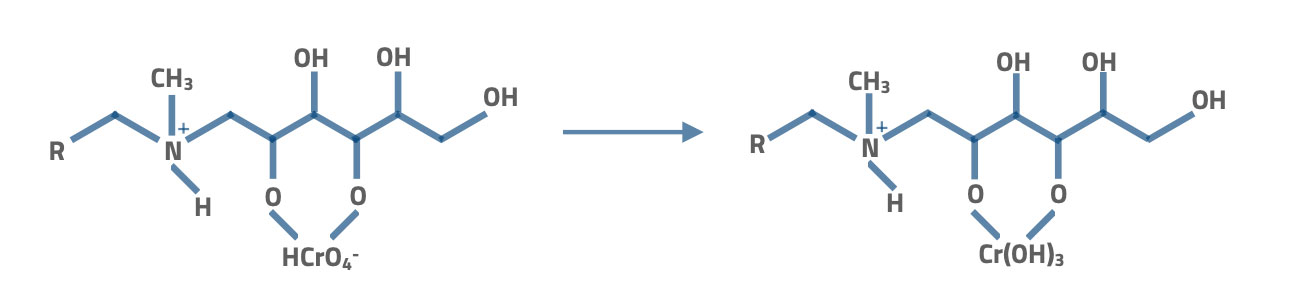
Also, these resins can remove Cr(VI) and As(V) ions. Here the sorption mechanism reduces the Cr(VI) into Cr(III).
Chelating resins will be successful in their performance with proper application conditions. Detailed information about the liquid that is going to be treated is essential. It helps to understand the metal concentration of the target liquid. The suspended solid level, Solution temperature, presence of oxidants, and organic contaminant availability will cause the resin fouling. Organic solvents such as alcohol, less-weight hydrocarbons, and ketones are inadequate for ion exchange. Still, the partial water solubles, such as different oil types, are not good at resins. Also the ions that build up complexes change the metals’ nature, and the presence of such ions, EDTA, chlorine, ammonia, and cyanide need to be aware.
Another two parameters are pH level and Total dissolved solids (TDS) concentrations in the inlet water. The chance to control the parameters will help the successful operation of chelating resins that has a narrow operation pH range.
Pay thorough attention to the wastewater generation process. With a well-known process, the ion exchange resin applicability is also well-known. It will stop under-design or over-design of the plant, and cost-effective, efficient treatment will popped-up.
Chelating resin technology is an appropriate technology with proper knowledge of basic details of effluent water that will be treated.
The chelating resins don’t like to operate in high flow velocities and are very flow-sensitive. The increasing bed depth provides a better operating environment. Please be keen on limiting the pressure losses through the bed not to exceed 20psi.
Chelate resins show poor sensitivity to the chlorine and suspended solids of the influent water. These resins will work appropriately with zero oxidant levels. Chelating resins offer a more extended run length, and with the higher turbidity levels, the fouling will be accelerated.
Organic solvents (polar and non-polar) don’t harm the chelating resins, but these solvents can form complexes with metals that are not attached to the chelating resins. Partially soluble polymers can be coated at the surface of the resins and prevent ion exchange.
Each chelating resin type has its operational pH range, but in common, resins are stable in pH ranges from 0-14. Some chelants don’t operate at high pH levels. Usually, the increasing temperature can increase the chelating resin capacity. Chelating resins are stable at least 140 0F.
Chelating resin regeneration can proceed as two main processes; Acid injection and neutralization. The chelating resin bed regeneration schedule has five steps as normal processes of ion exchange resins.
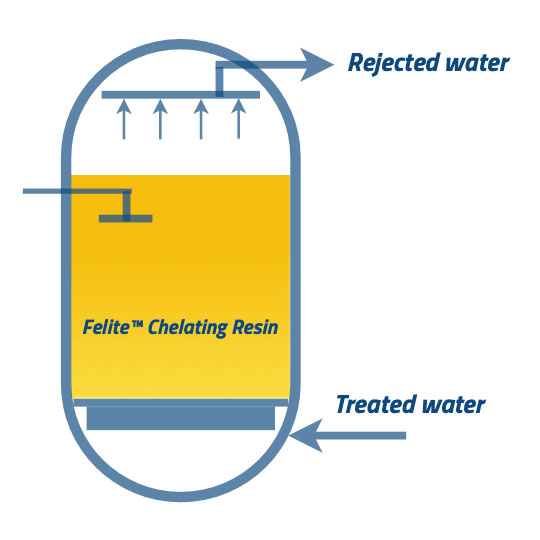
①, In this sequence, the backwash step helps to remove the suspended solids in the resin bed. Treated water is injected through a bypass line as an up-flow to redistribute the compacted chelating resin bed. The proper velocity of backwash flow removes the attached particles at the surface of the beads due to the agitation.
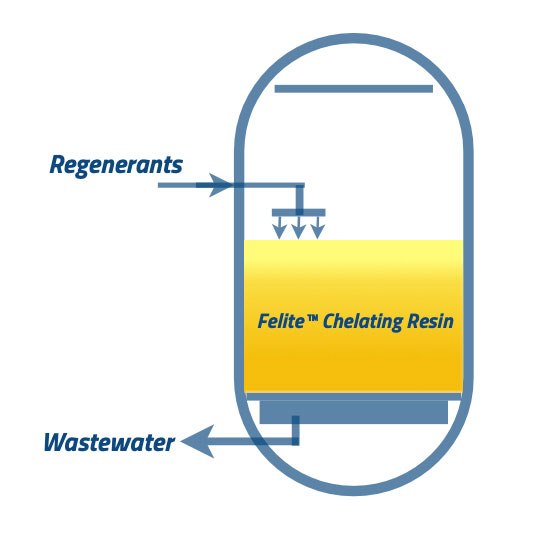
②, Regenerants are added to the chelating resin beds as two procedures; acid injection and neutralization. After the backwash process, acid injection is going to be applied. Most of the time, hydrochloric acid is used to strip the contaminants. But, in some cases, sulfuric acid is also involved. Hydrogen ions stabilize the chelating groups but couldn’t form ligand bonds in this situation. Acid pH should be able to spring the chelant bonds, but the resin can be non-selective for the metals in the acidic condition. Therefore, neutralizing should be brought to the stage to reactivate the chelating resins. Before introducing the base solution to the system, it should rinse the acid from the resin bed. The feed water line helps to flush the acid in the bed. After acid rinsing, the neutralizing step needs to be started. Also, the acid can dissolve some ions in the resin bed. Regeneration with sulfuric acid is complicated due to the metal sulfate precipitations.
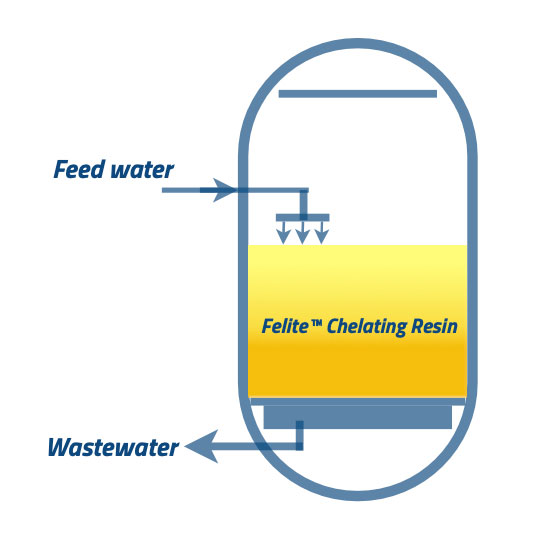
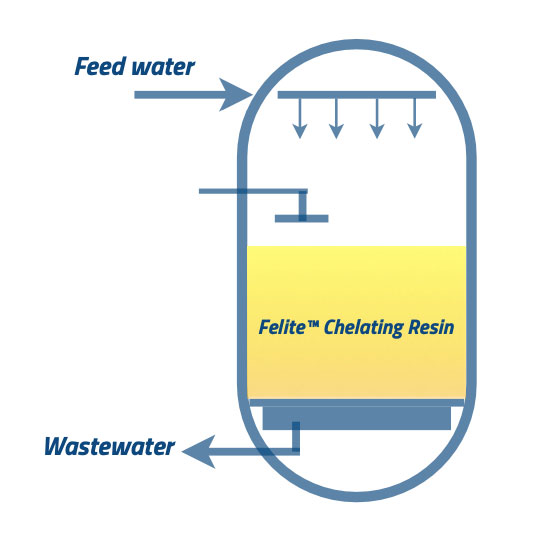
③ ④, Having a final rinse out of bed at any convectional flow rate and suitable timing is essential. The resin bed can neutralize with properly prepared caustic solutions, and it should also need to be rinsed out. It would be best to carry the final rinse until the conductivity, and the pH levels are at the permissible levels in the process. The feed water to the down-flow direction will remove the acids and base, and washout will be discharged through the wastewater line. You can carry this step as a slow rinse and the fast rinse. At the slow rinse step, the feed water will be inserted into the system at a similar flow rate of regenerant addition, and it should not harm the resin beads. Fast rinsing carries a similar flow rate to the service flow rate. it is continued until the treated water comes to its permissible levels.

Pablo Hensley - Supply Chain Coordinator

Alex Allman - Purchasing Manager