Water quality expresses the chemical, physical, and biological characteristics levels concerning its targeted purposes, such as drinking, processing, or swimming.
Alkalinity is a chemical parameter that expresses the acid and base neutralizing capacity of the water body or buffering capacity of water and maintaining its stable pH level. Alkalinity presents due to hydroxide ions, carbonate ions, and bicarbonate ions in water. In most cases, bicarbonates with carbon dioxide create bulk alkalinity in natural water. Alkalinity in naturally occurring water is a function of pH water.
Alkalinity in water combined with water hardness will be a critical contaminant in industrial water-consuming processes. It should be removed and under control to scaling & corrosion problems to reduce the operational and maintenance cost. Scaling potential is more significant in industrial heat-changing equipment due to higher heat transfer rates and higher operating temperatures. Water treatment specialists introduce new technologies to purify water. Alkalinity plays a significant role in boiler operations.
Effect of alkalinity in boiler operations
Influent alkaline water will generate hydroxide and carbon dioxide due to the breakdown of carbonates and bicarbonates in feed water during the steam generation process. Carbon dioxide vaporizes with the steam, condensates back with the steam condensation process, and forms carbonic acid. This highly acidic solution deteriorates the condensate return lines and fouling with the return crudes to the boiler. Hydroxide alkalinity supports further corrosion.
Removing alkalinity in industrial applications
Removing alkaline ions from water is called “dealkalization.” Some amine compounds can protect the condensate lines from corrosion, but it is not a cost-effective method. More amine is required with more alkaline feed water. The boiler operating process requires hardness removal and reduction of alkalinity with no removal of other solids. In the water treatment industry, process engineers prioritize membrane technology and ion exchange technology for dealkalization. In most cases softening doesn’t remove alkalinity, but demineralization does at a high cost. This article explains the types of dealkalization processes with ion exchange technology.
Ion exchange process for dealkalization.
There are three main types of dealkalization process that can identify.
- Chloride anion dealkalization
- Split stream dealkalization
- Weak acid cation dealkalization
1. Chloride anion dealkalization or salt splitting dealkalization
This method is the most common in commercial & simple industrial applications and where low-pressure boiler operations are conducted. The ion exchange process uses strong base anion type-II resins in the form of chloride. Chlorine dealkalizers are similar to the ion exchange softener units except for the resin type in the vessel. Also, dealkalization can use as a combined system after a softener unit. This anion unit can replace carbonates, bicarbonates, sulfates, and nitrates. Follow the below equations to have a clear idea of ion exchange. “R” represents the “resin.”
Na2SO4 + 2R – Cl → 2 NaCl + 2R –SO4
2NaHCO3 + 2R – Cl → 2 NaCl + 2R – HCO3
2Na2CO2 + 2R – Cl → 2 NaCl + 2R – HCO3
2NaNO3 + 2R – Cl → 2 NaCl + 2R – NO3
The salt splitting de-alkalization process has 90% efficiency in reducing the alkalinity in water without lowering the total solids. Reducing alkalinity decreases the carbon dioxide (CO2) in boiler condensate resulting in a low concentration of amine. Due to the stable TDS amount in alkaline water, there is no increase in the boiler’s concentration cycles. There can be slight increases in conductivity in boiler feed water.
The moment of the rapidly increased value of the treated water alkalinity indicates the resin bed’s exhaustion and signaling for a regeneration process. The regeneration process can follow two types of solutions. The first one is to use sodium chloride or brine solution to regenerate the resin bed. A salt-caustic combined solution can be used as the second regenerant. Better to use evaporated graded salts in high-quality levels for regeneration.
There is a chance to convert excess softener capacity into the dealkalizer. Salt splitting de-alkalizer performs few disadvantages.
- The dealkalizer has low operating capacities; therefore, higher operating levels need large vessels, a large number of resins, and a high level of regenerants.
- The process is unable to reduce the TDS level when required.
- The emergency flow of hardness ions and organics through the dealkalizer may cause resin fouling.
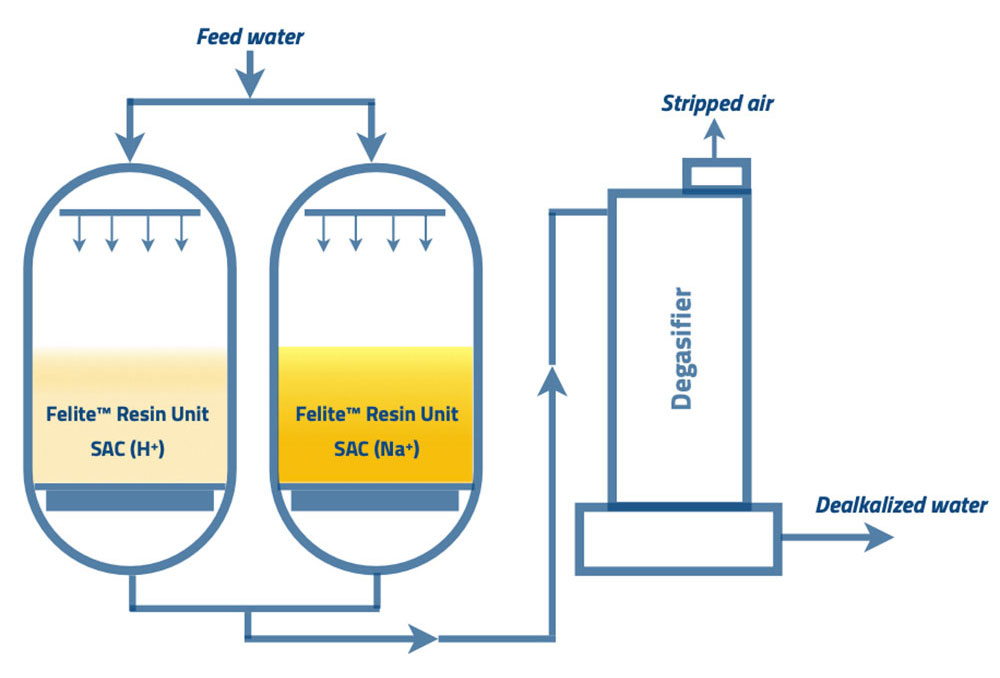
2. Split stream dealkalization
Alkaline water passes through the two parallel units of strong cation resins. One vessel is filled with cation resins in the form of sodium, and the other vessel is filled with resin in the hydrogen form. Then the effluent water from the parallel units combines. The effluent water from the hydrogen form resin bed consists of carbonic acid and free mineral acids.
The effluent water from the sodium form of the cation resin vessel contains carbonate and bicarbonate alkalinity. When these two streams are combined, the free mineral acids convert carbonate and bicarbonate alkalinity into carbonic acid. The carbonic acid is unstable in water and forms carbon dioxide and water. The water with carbon dioxide passes through a degasser or a de-carbonator. The degasser strips the carbon dioxide with an up-flow air stream.
Mixed water alkalinity can be controlled by maintaining the percentages of two effluent water while blending. Water from the sodium form cation resin in higher percentages results in high alkalinity, and water from hydrogen form resin in higher percentages results in low alkalinity. Therefore, the final effluent water alkalinity can be controlled by the split stream dealkalization process. Boiler cycle concentration and the boiler operation process can be affected by the high alkalinity in feed water. Hence, reducing the TDS and removing alkalinity through this process is the best advantage.
There are positive points in the split stream method, and they are;
- Final effluent water alkalinity can control up to the desired level.
- Both alkalinity and TDS can be reduced.
- Required resin volume is lower than type-11 due to the higher operating capacity of strong acid cations used in the split stream process.
Also, there are some downsides to this method;
- Generally, SAC resin beds can regenerate with sulfuric acid, and the regenerant is hazardous.
- A small caustic concentration may require stabilizing the pH level in the final treated water.
- Extra operational and maintenance costs for the degasser.
3. Weak acid cation (WAC) dealkalization
Usually, engineers perform the weak acid cation dealkalization process for equal concentrations of hardness and alkalinity in ppm as CaCO3 or hardness to alkalinity ratio one or more. Consider water has higher alkalinity than hardness; alkalinity is not removed to its lowest level. When hardness exceeds alkalinity, slight hardness can remain with the treated water. Therefore, the treated water with slight hardness needs a polishing plant (a softener plant) for further purification.
The dealkalization process with WAC resins is highly efficient and cost-effective. The resins are in the form of hydrogen. There should be a decarbonater to remove the carbon dioxide in water prior to the polisher. Adding a small amount of caustic solution after purifying may require adjusting the final effluent pH level.
WAC dealkalization method offers higher operating capacities and low regenerant cost due to high efficient WAC resins; it requests a small amount of regenerants. Also, a small softener capacity is needed due to the removal of bulk hardness with WAC, reduced TDS, and less wastage in regeneration. Also, there are some identified drawbacks to WAC de-alkaline system. They are;
- Not efficient with sodium chloride alkalinity.
- Using sulfuric as the regenerant is hazardous.
- The capital cost and the O&M cost are required for the decarbonator or degasser.
Conclusion
- The ion exchange process is an efficient and cost-effective technology for the dealkalization process. Removing the alkalinity is called, dealklization.
- The alkalinity of water is the buffering capacity of water. Alkalinity protects the ecosystem with unstable pH conditions in the water body.
- Human consumption of high alkaline water in commercial applications may cause many critical conditions. Therefore, reducing alkalinity is essential.
- Alkalinity with hardness results in scaling and corrosion in heat transfer equipment in industries, especially in boiler operations. Water treatment experts recommend ion exchanging methods for water purification.
- There are three different dealkalizers;
- Chloride anion dealkalization
- Split stream dealkalization
- Weak acid cation dealkalization
- You must be able to select the best dealkalizer with the help of your water treatment engineer.