Home » Knowledges » Mixed Bed (DI) Resin Regeneration Process

Mixed Bed(DI) Resin Regeneration Process. Deionization (DI) is highly practicable in water treatment technology. Deionization removes dissolved solids in water. The ion exchange technology involves in the DI process. There are many soluble anions and cations in water that need to be removed. An ion exchange system exchanges their H+ and OH– negative and positive ions, and ultra-pure water results.

Some many technologies and industries use Mixed Bed(DI) Resin Regeneration Process technology. Some are insulating/cooling, pharmaceuticals, electronic industries, power stations, dialysis, wastewater discharge, laboratories, etc. DI water system is a combination of several DI units or one DI vessel and other components to produce purified water. Depending on the requirement, a system can be simple with one unit and complex with several units using RO, NF, or UV filtration. According to the design, DI water purification plants can be duel bed systems or mixed bed systems.
Deionization using an ion exchanging system is the standard method used worldwide. It is an efficient, user-friendly, cost-effective system that produces ultra-pure water.
The common ion exchangers are resins. Experts use different types of resins as per their requirements for water polishing. We can identify ion exchangers into two main sectors; Natural and synthetic. There are inorganic and organic resins in each sector. Some examples of natural inorganic resins are Zeolite and Vermiculite. Usually, synthetic organic resins are the most abundant resin type within the field. They are plastic beads produced with polystyrene crosslink with divinylbenzene.
The plastic beads contain charged anions and cations. The beads with the negative charge are anion resins, and the resins with a positive form are cation resins. Cation resins are present in the form of H+ in deionization, and anion resins are present in the form of OH–. These cations released their cations into the water and captured the cations in the raw water. Anion resins do the same to the negatively charged particles in raw water.
Strong acid cation (SAC) resins and strong base anion (SBA) resins are the prominent resin types used in deionization plants. SAC is created by adding the SO3-H group, and the SBA is with a quaternary amine group. In some cases, the weak base anion is also used with SAC, and car washing spot-free rinsing is a good application. The mixed bed DI plant contains SAC and SBA resin mixer in one single bed.
When the resin beads continue their exchanging process, the resin bed gradually saturates with the dissolved contaminants in raw water. The effluent water shows increasing conductivity as a failing DI unit indicator. Therefore, it is essential to refresh the resin bed for efficient work. Releasing bonded contaminants from the resin bed and attaching the relevant resin forms by exchanging methods is regeneration. There are differences in the regeneration of duel bed Di plant and mixed bed DI plant.

The regeneration of anion and cations in the DI duel bed plant is complicated. The regeneration process includes strong acid and base; therefore, you should follow the appropriate safety conditions in handling chemicals and removing generated waste.
The active ions, Hydrogen in the SAC column, exchange the positive ions such as Mg2+, Ca2+, Na+, and the SBA unit exchanges sulfate, chloride, alkalinity, and silica with their active ions, Hydroxides. Reducing the concentration of available active ions due to ion exchanging and becoming to a lower level is considered as resins are exhausted. When the conductivity setpoint indicates endpoint leakage, regeneration should be carried out.
We can identify a few steps in this process;
The key point of backwashing is to loosen the compacted resin bed. The resin bed collects suspended impurities and some broken fine media particles (beads) during its service cycle. In the opposite direction of the service cycle, a calculated flow rate appropriate to the given specifications of resins loosen up the resin bed and expands up to its freeboard. This water flow removes fine particles, reduces the likelihood of channeling, excessive pressure drops, and loses its compaction. Please refer to the consultant specification sheets given for anions and cations due to quite different densities of the two resins.
Strong acid cations need to be regenerated with sulfuric or hydrochloric acid (HCl). Strong base anions are regenerated with Sodium hydroxide or caustic soda. The strength and flow rate of diluted regenerant is essential to prevent resin fouling.
Acid solutions supply H+ ions to the resin bed. The solution substituted H+ ions to the bed and stripped out the positive ions attached to the resin beads. Typically, HCl concentration varies from 4% to 6%. The application of sulfuric acid needs to be calculated depending on the calcium ion concentration of the water to avoid the precipitation of calcium sulfate. The sulfuric addition should be done with supervisory advice. The contact time of acid is around 30 minutes.
Hydroxyl ions in a caustic solution stripped negative ions in water and substituted OH– into the resin beads. 4% concentration is commonly used in anion regeneration, and a contact time of 45 to 60 minutes is preferred. Temperature and the contact time need to be concerned due to silica availability and its amount.
Slow rinse needs to be similar to the flow rate of diluted regenerant introduced. The displacement of regenerant from the systems starts with this step.
The flow rates should be introduced to the system at a higher rate than slow rinsing to remove excess regenerant from the system. The resin beds are in the activated form and will be ready to start the regular service run.

Mixed bed (MB) resin combines SAC and SBA in a certain ratio( normally 2:3 ). The resistivity or electrical conductivity is the only way to measure the mixed bed water because it is deficient in dissolved ions. The main point of success of the MB unit is continually working to neutralize the pH values. MB resins can deliver high-quality water with higher capacities to the given quantity of MB resins than the two-bed resin DI system.
Regeneration is sensitive and complicated compared to duel bed resin regeneration. The steps in mixed bed resin regeneration are;
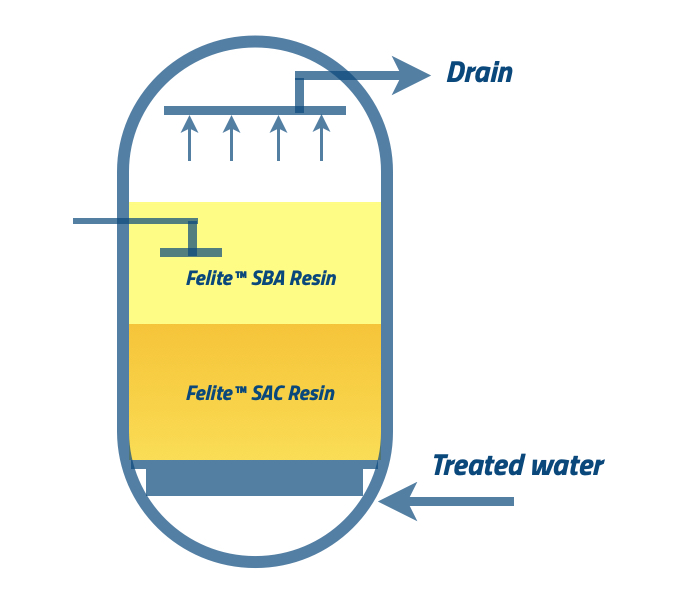
Backwashing is the first step, and backwashing the exhausted MB results in two separate anion and cation phases according to their density. The cation resin has a higher density than anion resin; therefore, cation resin goes down, and anion resin goes upstream. Backwashing flow rates need to be adjusted to have enough bed expansion. Also, anion resins can be entrapped at the bottom layer; therefore, changing flow rates helps prevent accumulation at the cation resin layer. It is necessary to start with a high flow rate for fluidization of the resin bed, and reducing the flow rate will prevent the anions from escaping.
Backwashing can be conducted within 20-30 minutes, but it can be 40 – 50 minutes with the heavy suspended solid load.
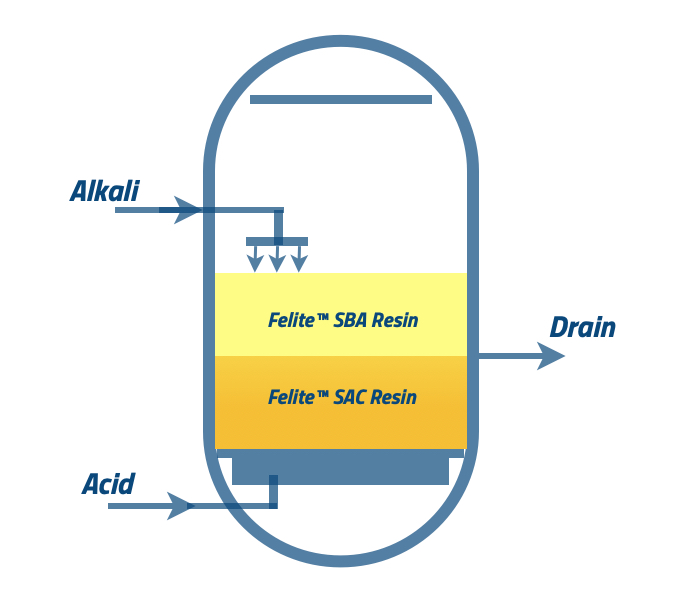
Caustic soda (NaOH) is a commonly used regenerant for anion resins, and hydrogen chloride (HCl) is the regenerant for the cation resins. NaOH is injected from the top of the vessel and collected from the middle collector. HCl is injected at the bottom of the line and collected from the middle collector. Usually, the injection time should be 20-30 minutes. It would be best if you used prescribed concentrations for acid and base.
Regenerant injection can be arranged in “simultaneous injection” and “sequential injection.”
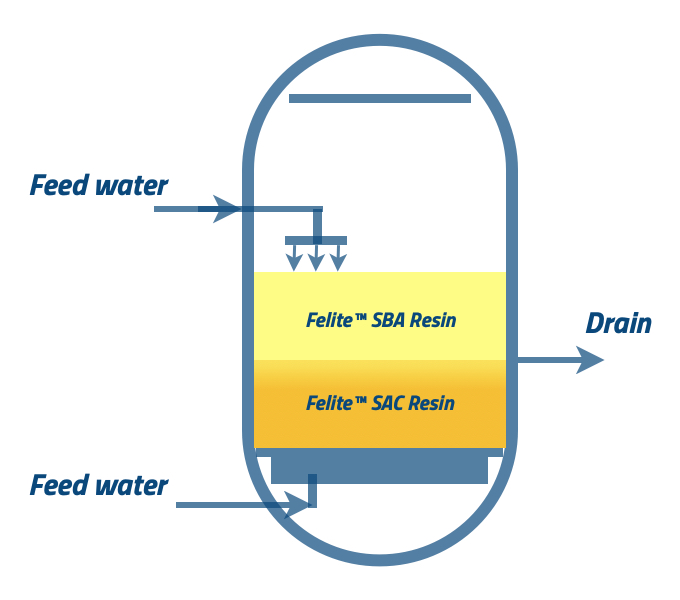
When you complete the regenerant introduction to the resin beds, continuous water flow is required to displace the regenerants.
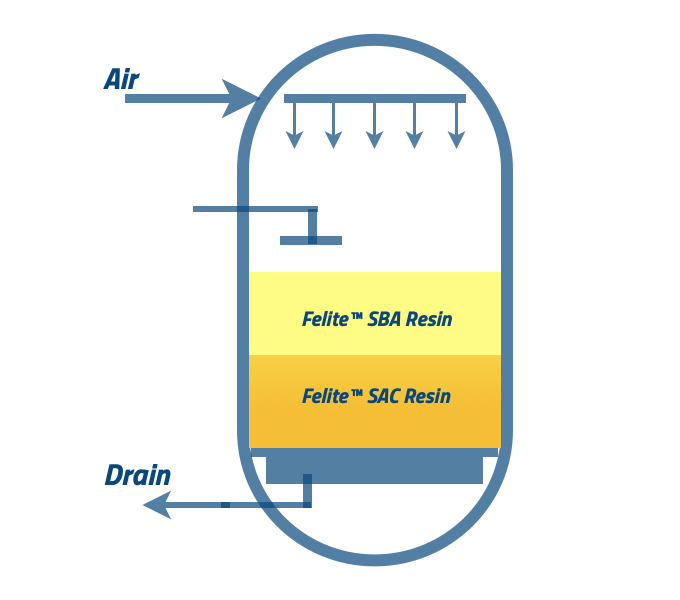
The water in the shell needs to be drained before occurring air mixing. It will help to have a homogenous mixing of both anion and cation resins. The water is requested to drain down up to the level of the resin bed. Please follow the suppliers’ guidelines.
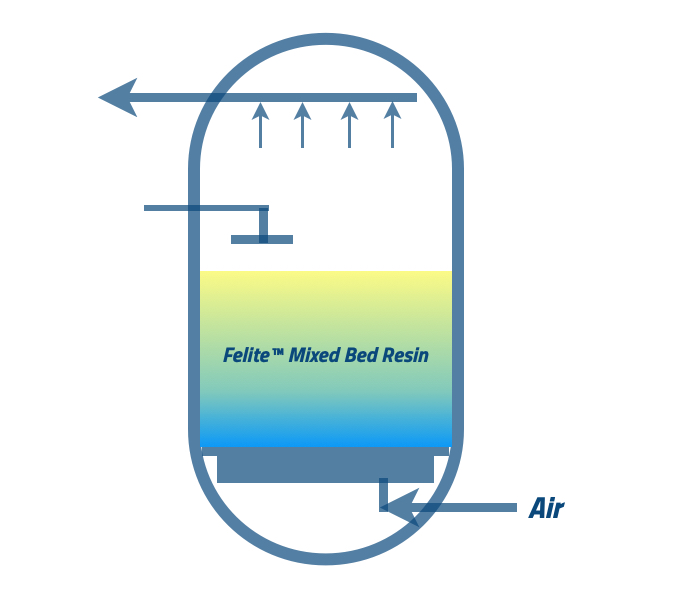
The recommended airflow help anion and cation resins beds mix with nitrogen or fresh air. There is a chance to trace oil when you use a compressor. Mixing time can be >5 minutes and less than 15 minutes.
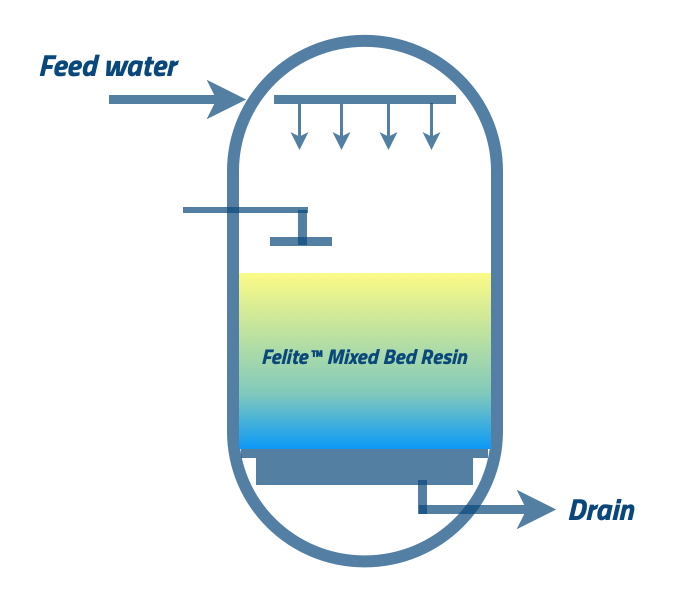
This step also can identify as the fast rinsing, and it is used to complete the regeneration procedure to obtain the required effluent quality of the DI plant.
Now, your DI resin is ready to go.