What are silicates?
Silica is one of the most abundant elements contained in natural water bodies. The chemistry of silica is quite complex as carbon compounds and second only to carbon complexes. Silicates are a large group of a combination of Oxygen and silicon in a tetrahedral structure ( SiO44-). Silicate minerals have many formations, and they are everywhere. Silicon dioxide, silicate anions such as orthosilicates, metal silicates (Zn2SiO4, Fe3Al2 (SiO4)3), mica, gemstone, talc, feldspar, and zeolite (alumino-silicates) are some examples for silicate formations. The main three isotopes of silicates are non-radioactive. Silicates are used in cement, electronics, ceramic, glasses, fireproofing papers, woods, detergent formulations, and pottery industries. Alkali silicates are used in the glue industry.
Can Silicate Removal dissolve in water?
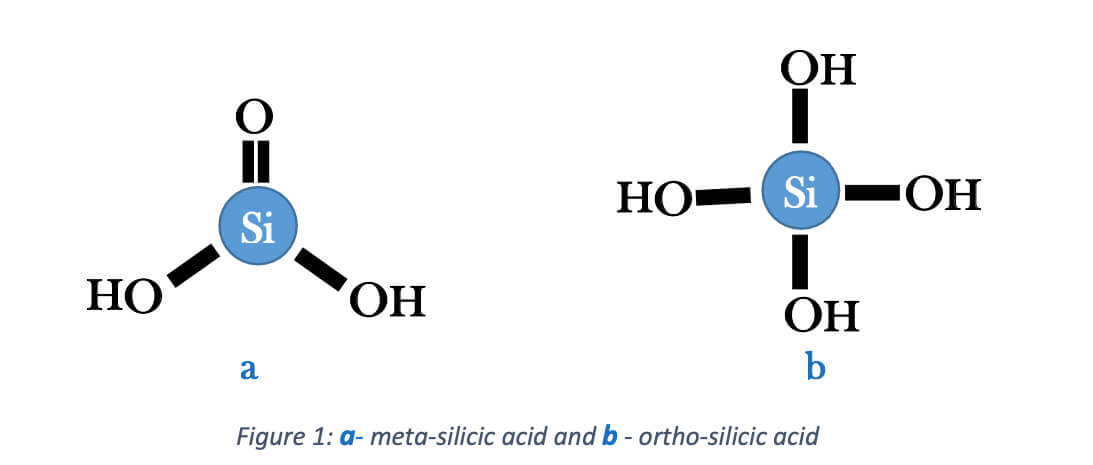
Silicate is a vast field to discuss, but this article mainly focuses on the silica that dissolves in water and the removal of silicates using resins. Chemical engineers in water treatment fields write aqueous silica or dissolved silica as SiO2. The reason is that the solid silica deposits contain one silicon mole per two oxygen moles, and some compounds, such as quartz, slightly react with water and generate silicic acid. That reaction can form ortho- or meta-silicic acid by the following hydrolysis. And it is in the equilibrium of bisilicate. Silicic acid in the ortho model is helpful in water treatment and highly soluble in high pH values.
SiO2 (s) + H2O (l) ←→ H4SiO4 (s)
H4SiO4 (s) + H2O (l) ←→ H3O+ (aq) + H3SiO4– (aq)
Many silicon compounds react with water, such as silicon tetrafluoride and silicon tetrachloride. The solubility of silicon compounds is different for each. But silicon carbide is insoluble in water.
Silica also can exist as colloidal silica or as a polymer. These are present in long-chain and have no ionic form; therefore, these non-ionic particles can’t remove with the same technology for reactive silica.
What are the health and environmental aspects of silica?
Silicon is an essential element in plant growth. Dandelions and bamboo plants contain silicon in their stems and leaves. Also, silicon can be identified as a dietary requirement in many organisms. Chicken & mice need silicon to develop their bones. Silicon supplements use as a medical treatment for humans to strengthen bones (avoid osteoporosis). Also, tissues and skin contain a significant amount of silicon in the human body.
Silicon is generally identified as harmless when present in water. But, abnormal and elevated levels of silica in water sources may limit the algal growth. Zeolite is able to replace phosphate in detergents. Therefore, water organisms can be affected.
The silicon halogens are very toxic and corrosive. Silicon tetrachloride is a skin and eye irritant and can result in breathing problems.
Is there any silica removing water treatment methods?
Higher levels of silica are a problem for the boiler and cooling tower systems. High pressure boilers will have silica vapor due to its enough volatility in the system. At the reducing pressure of turbines, the silica vapor is deposited on the turbine blades and reduces the turbines’ efficiency. Both colloidal and reactive silica causes; reactive silica breakdown and will vaporize at high pressure and temperature. Elevated levels of silica are also a problem with heat exchangers in cooling tower systems. Silica can present a monomeric form when pH > 10, which is highly soluble. The monomeric form can condense with decreasing pH levels and be converted into silicate complexes by sharing OH-.
The silicate removal systems are designed to protect the boiler systems and cooling towers from corrosion due to condensing silica on those system components. There are several methods to remove silica in water.
Some of them are;
- Coagulation and flocculation remove the colloidal silica, but it removes a small fraction of total silica in raw water. Precipitation methods are cost-effective methodologies of partial removal of total silica. But there are many drawbacks, such as time-consuming, messy silica precipitations, and challenging to accomplish. Silica does not precipitate as SiO2. Silica can always contain divalent ions and rarely can be trivalent ions. Calcium silicate is insoluble. At high temperatures, they have rapid formation. Though aluminum compounds can be used to precipitate silica, the conventional compound is magnesium salts.
- A membrane system like Reverse Osmosis (RO) helps to reduce soluble silica concentration, but not entirely. It removes various forms of non-soluble silica in water. In RO systems, new polymers are rejecting silica. Hyper filtration is the removal mechanism, but it relates to silica ionization because the silica is completely dissolved in higher pH values. Designing a RO plant needs more concern about the concentration of the silica in brine solution and the solubility of silica.
- Ion exchange (IX) technology is the best method that uses incomplete removal of soluble silica. The following subtitle will further explain silica removal with an ion exchange system.
Does an Ion exchange system used for Silicate Removal?
Ion exchange technology is famous worldwide due to its high efficiency to the target, low operational cost, easy maintenance, user-friendly, and low initial cost. Resins play a leading role in an IX system, and the total function of the system depends on the activity of the resin bed.
In general, there are mainly two types of cations and anions. It can divide into four, according to the coated functional group to the polymer bead. They are strong acid cations (SAC), weak acid cations (WAC), strong base anions (SBA), and weak base anions (WBA).
Engineers select strong base anions for silica removal in water. SBA resins convert all salts into their relevant base. SBA resins consist of quaternary ammonium groups and are divided into two; type-1 and type-11. Lots of experiments are conducted under the silicate removal section. The SBA resin beads coated with hydroxyl groups have a higher preference for sulfate & chloride than silica but higher than for hydroxide. But, SBA resins effectively remove reactive silica and reduce the silica concentration to ppb (parts per billion).
SBA resin bed is regenerated with the NaOH solution. The essential parameters of effluent water are effluent silica level and conductivity. NaOH can result in anions and will be a cause for small leakages of silica. The capacity of leakage depends on the dosage of regenerant and the chemistry of processed water. Silica can be removed from the resin bed with heated sodium hydroxide up to the given temperature by the manufacturer.
However, we couldn’t remove colloidal silica through ion exchange resins due to no charge particles in colloidal silica. The best method to remove that colloidal silica is filtration. Membrane filtration and UV filtration technologies are effective.
Conclusion
Silicon can be identified as the second most abundant element after Oxygen on the earth. We can find a massive number of silicon combined compounds in different formations. Silicon can be identified as dissolved compounds in water, and the form is SiO2.
Silica removal is essential in boiler and cooling towers. The best technological method uses an ion exchange system with a filtration method. Complete removal of silica will helps to secure the boiler and cooling tower systems with a low operational cost.
