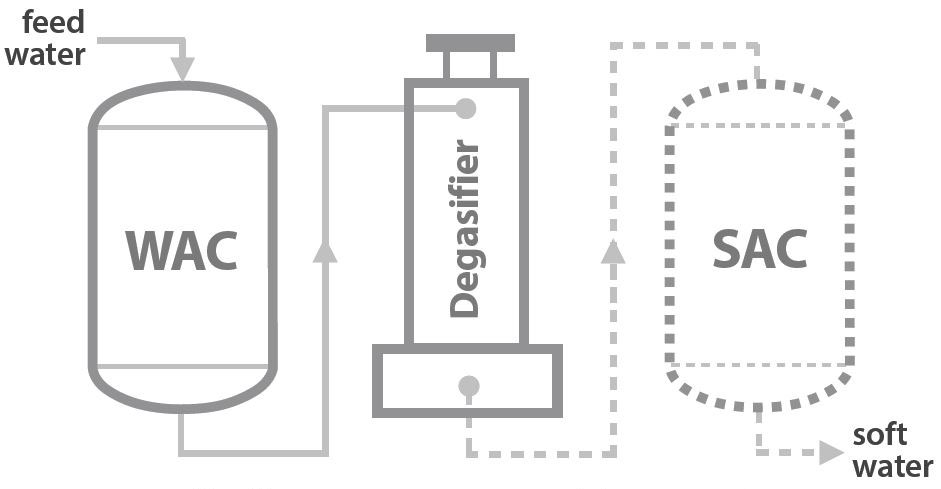
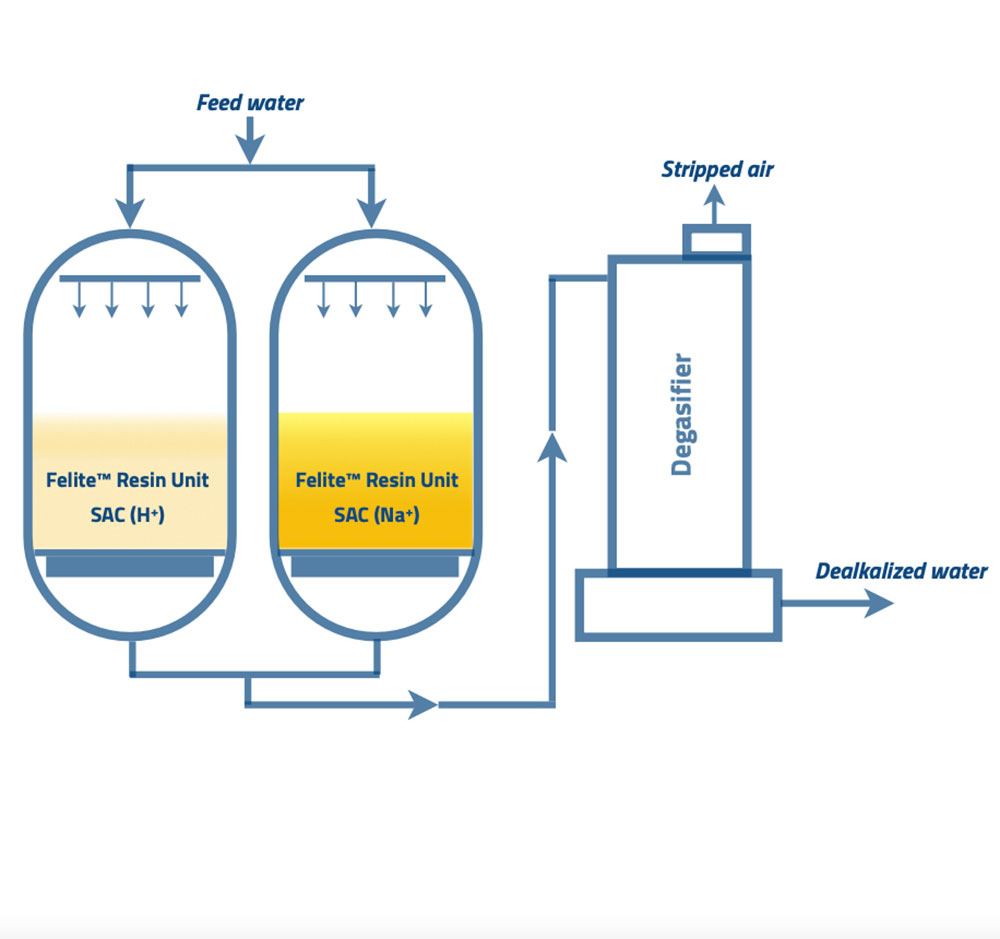
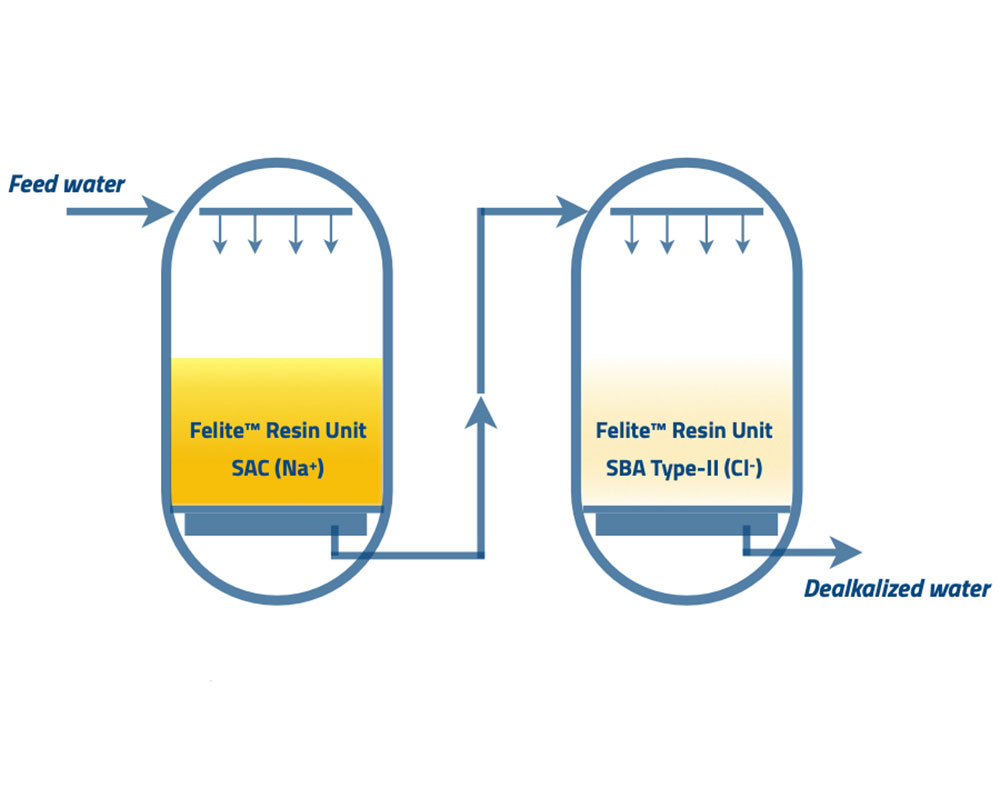
Resin Category
Weak Acid Cation
Polymer Matrix
Polyacrylic Acid, Macroporous
Ionic Form
Hydrogen
Total Capacity
4.7 min. (H+)
Particle Size
0.3 – 1.2 (16 – 50 US Mesh)