Before we talk about softener resin regeneration, do you know why we use a water softener?
The Water softener helps remove calcium and magnesium ions (hardness) in your well water. And it removes manganese and iron up to some degrees. Softened water reduces the deposits of cations carbonates surfaces of pipes and heat exchange equipment. Raw water with hardness makes lots of issues to boilers, cooling towers, and laundries. Water softener plants are very cost-effective methods for softening in the industrial field to solve these problems.
Following the introduction of the ion exchange procedure will expand your understanding of the regeneration of softener resins.
What is the Process of Ion exchange?
A water softener always utilizes the ion exchanging process, and resins are the main component in this process. Resins are synthetically manufactured polymer beads that have electrostatically bonded with functional groups. According to these functional groups, there are four types of resins called Strong Acid Cations (SAC), Week Acid Cations (WAC), Strong base Anions (SBA), and Weak Base Anions (WBA). Functional groups of SAC and WAC are the Sulphonic group (-SO3H) and the Carboxylic group (-COOH), respectively. SBA holds quaternary ammonium groups, and WBA consists of primary, secondary, and tertiary amine groups.
In the softening process, we use cation resins due to hard water that includes cations (Ca2+ and Mg 2+), so this article will explain cation resin ion exchange and its regeneration.
The Sulphonic and Carboxylic groups interact with water-soluble ions such as H+, K+, and Na+. The affinity between these groups and ions express through electrostatic bonds. Anionic functional groups catch positive ions and vice versa. The electrostatic bond between ion and the functional group is comparatively weak. The following image will clearly explain the ion exchange.
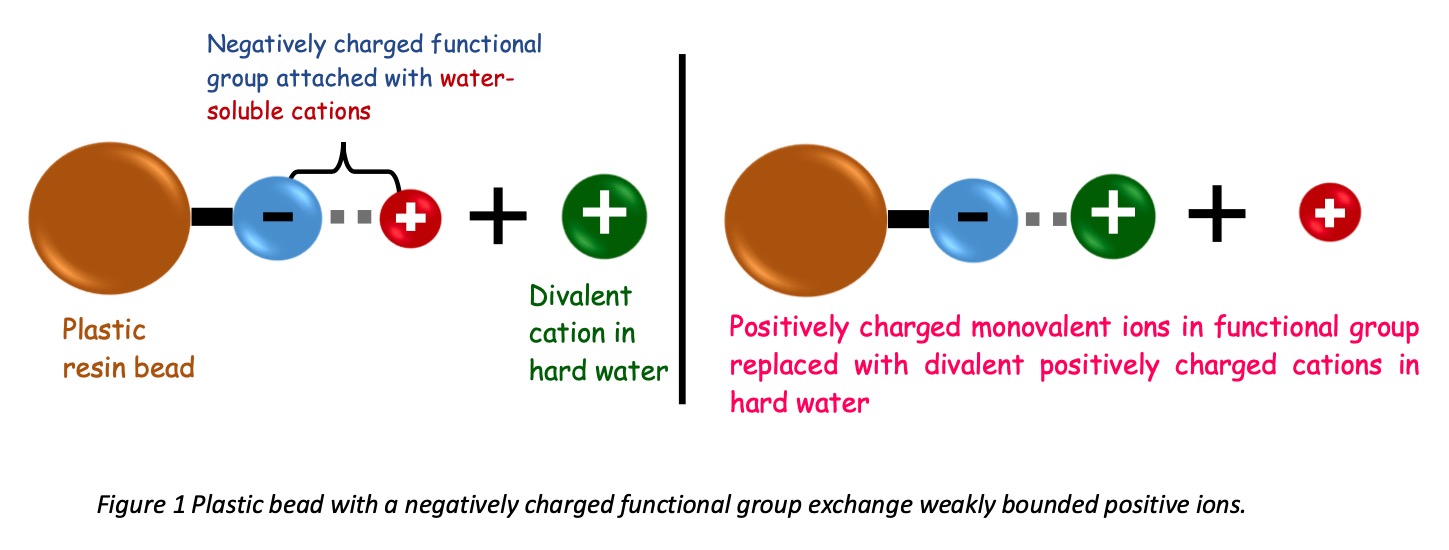
Within the industry, the softener resins pre-applied with sodium ions. While softening, hard water cations take the place of Sodium and release sodium ions and salt to softened water.
Now you know how resins are essential in water softening.
Why do softener resins need regeneration?
Continuous operation of softener may cause for saturation of resin bed. That means the continuous ion exchange process affects complete attachment with hard water cations and no more ability to bind Ca2+, Mg2+ to the resin bed. So hard water passes through the plant without treatment. What can we do about it? Do we need to replace resins? No, it is not necessary. Some issues are occurring that need to replace. But when saturation occurs within the bed regenerating is more effective than replacing. It is cost-effective with cheaply available regenerate agents and a straightforward method. So, experts in the water treatment field design a time-to-time regeneration process to protect the resin beds and increase the long life of these plastic beads.
Do you know the regeneration process?
The word “regeneration” clearly expresses the idea of the process. When freshly generated polymer beads end their performance, we regenerate them into fresh and high-performing resins using different methods. In almost all the softener plants, four common significant steps proceed during the regeneration service cycle. They are; Backwashing, Brine injection, Slow rinsing, and fast rinsing. This softener service cycle for regeneration concludes an automatic operation.
Backwashing
Water has a downward flow through the softener plant in the usual procedure. Resin is a perfect ion exchange media as well as it works as an excellent filter media. Therefore, the downward flow of the water trap accumulates fine contaminants particles in the resin bed. In backwashing, water passes upward from a water distribution pipe at the bottom to the top through the resin bed. This up-flow rub the beads each other to clear precipitated fine particles, damaged beads, and resin fines. The upward flow cause dislodging and reclassification of the resin bed. This routine brings finer resin beads to the top and settles larger particles to the bottom. This specific process is very effective because it enhances the uniform circulation of regenerated chemicals (Brine solution and service water.
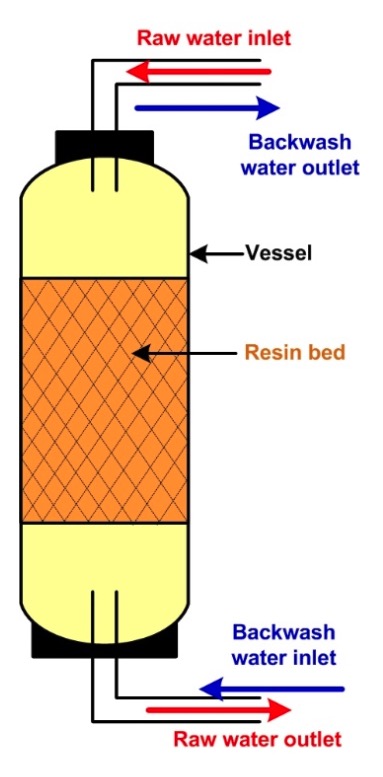
Insufficient backwash water flow causes fouling and channeling, while excess water flow cause for loss of resin beads. The resin bed washing should be continued until the backwash outlet gets clear. It usually takes 10 minutes. But time depends on water quality. The flow must be sufficient for the expansion of resin beds and is affected by the temperature. Also, it must be checked regularly for having a proper bed expansion. It will help if you take appropriate advice from your plant designer.
Designers use the treated water line in backwashing and release washed water with suspended solids though no chemical changes. This outlet can return to the clarifier or the influent tank.
Adding brine solutions
Releasing a regenerated brine solution to the resin bed after backwashing is called regeneration. The brine stream enters through a separate distribution line to the vessel. It has a slow downward flow which collects all hard cations into the underdrains. The concentration of brine solution should be strong enough to break down the attached hardness cations. In common, 10% NaCl is used, and it should be contacted until it allows to complete replacement of Na+ with hardness ions.
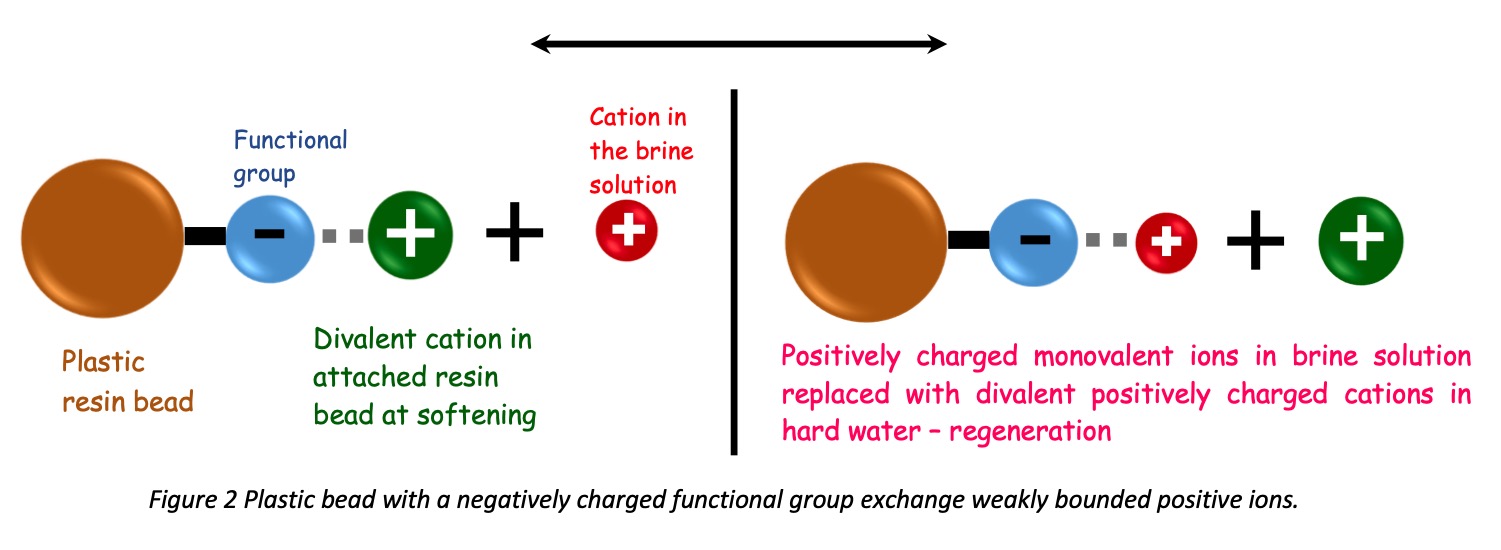
As shown in the picture, once the resin bed gets saturated and the brine solution enters, Ca2+ and Mg2+ ions exchange their places with Na+ ions in the brine solution.
Slow rinsing
This rinsing needs to flow continuously throughout the brine solution distribution system. The Slow rinsing step completes the regeneration by having a proper contacting regenerate agent. Usually flow rate of displacement water (slow rinsing) is the same flow rate of dilution of concentrated brine. Soft water is used for rinsing and helps completely move brine through the resin bed gradually.
Fast rinsing
In this step, water enters the bed with a high flow rate after completing displacement rinsing. Water removes excess brine and remaining residuals if any. Initial rinsing effluent contains a large amount of Sodium Chloride and hard water. But they are gradually decreasing. During the service cycle, the fast rinse must ensure high effluent quality.
Usually, in the resin regeneration process above four steps are completed. Complete ion exchanging, the resin bed is in equilibrium.
When to regenerate?
The period of water softener regeneration will be calculated depending on several data.
- Consumed water capacity
- Hardness level in raw water
- The iron concentration of the softener
- Resin tank capacity
For commercial purposes, regeneration can occur either complete saturation of resin bed or incomplete saturation of resin bed. Regular renewal will help expand the resin bed efficiency and increase the lifetime of resins. In most cases, regeneration is adjusted every day, taking about two hours.
If you have a twin-tank softener unit, the regeneration process takes a different way. When one vessel is going to be regenerated, the other vessel continuously provides the softened water and vice versa. Hence soft water is always available. The twin softener system increases the lifetime of the system.
Conclusion
Hard water is available in both aquifers and surface water. Due to some climatic changes, geological structures, such as lime dolomite dissolve and infiltrate aquifers and surface water.
Hard water contains calcium and magnesium cations (hardness in water) which cause terrible issues in residential and commercial uses. The main object of a softener is to minimize them.
Water softening includes an ion exchanging method for eliminating hardness. Most time, cation resins are used to remove hardness. Functional groups attached to plastic beads classify the type of resin. Here beads electrostatically bonded with the carboxylic or sulphonic group, which holds monovalent cations with weak bonds. So they exchange cations.
Exchanging hard water ions with resin cations will be saturated. Then there are zero efficiencies on hardness removing. So resin bed needs to be regenerated.
The regeneration process mainly consists of four steps: backwashing, regeneration, slow rinsing (displacement), and fast rinsing.
Backwashing has the upward flow to dislodge the resin bed. It helps to remove precipitated broken resin particles and finer particles. It also classifies the finer resin to the top and larger resins to the bottom.
In regeneration, the step added brine solution to the resin bed until complete saturation of resin bed with the cations in a brine solution.
Two rinsing steps ensure the complete exchanging of brine cations and excess brine removal. Slow rinsing occurs first, and fast rinsing should be done.
There are some negative benefits to regeneration. Discharge of waste of regeneration is a problematic issue. Some designers add them to sewer pits, but it will be a problem in industrial-scale plants due to backwashing large amounts of hard water, and saltwater can be released. It will cause digestive bacterial in the sewer. Also, discharge of this waste to the environment is prohibited, so researchers are trying different methods to solve this problem.



