We can find lots of ions in water. Electrically charged atoms or molecules can be interpreted as ions, and they can be negatively or positively charged. Negative ions are called anions, and the others are cations. Natural water with dissolved anions and cations is problematic and hence cannot use directly in various applications.
Deionization makes ultra-pure water by removing dissolved impurities from water. Some examples of usage of DI water are laboratory applications, pharmaceutical industry, petrochemical processes, food & beverages industry, boiler feeding, cooling applications, aquariums, automotive and electronic equipment manufacturing, etc.
Do you know about deionization? Let’s go ahead.
Do you know the deionization process and key role of resins?
Deionization or demineralization is a chemical process that forms high purity water by removing all charged particles in raw water using an ion-exchange method. Demineralization or deionization produces water that is similar in quality to distilled water.
As usual, resins play their leading role in the ion-exchange process. Resins are polymers manufactured with styrene crosslinking with divinylbenzene. Resins have electrostatically bonded ions. Resins with positively charged ions are called cation resins, and resins with negatively charged ions are called anion resins.
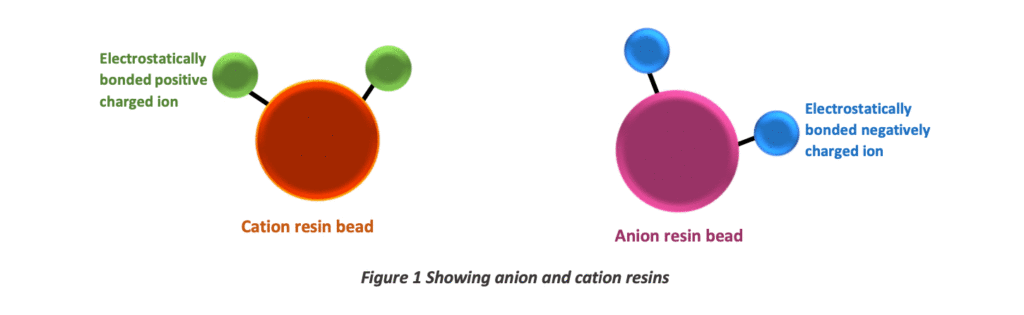
A deionization plant can consist of one or more resin columns. One contains strong acid cation, and the other has strong base anion resin beds. The cation resins exchange H+ ions for the cations in raw water. The anion resins exchanges OH– ions for the anions in natural water. The following image will clarify the exchange process.
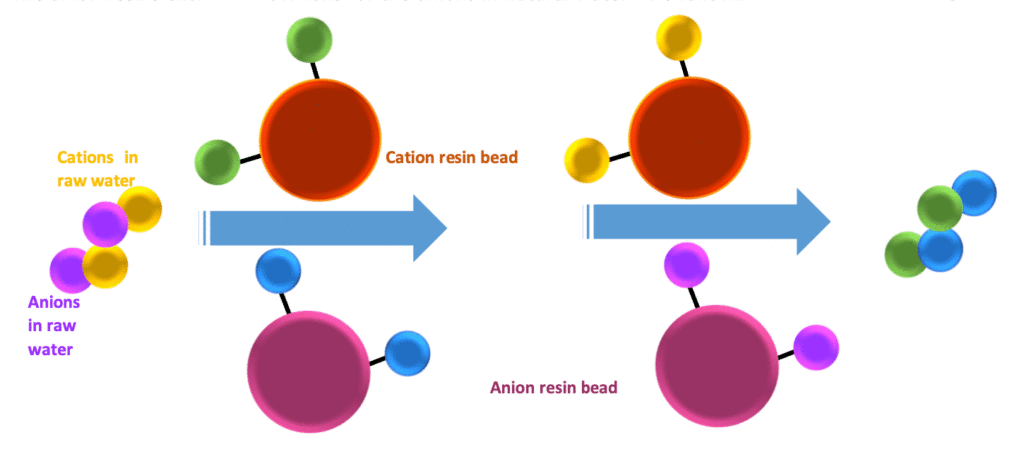
What happens within the cation resin column?
Lets’ get clarified furthermore. Imagine raw water contains Ca2+, Mg2+, Na+, HCO3-, SO42-, Cl–, and NO3– ions. As mentioned in the above paragraph, cation resins capture positive ions contained in this water and release H+ ions into the water. The anions in water bonded with released H+ ions and produced H2CO3, H2SO4, HCl, and HNO3. Strong acids are generated in effluent water from the cation resin bed. We can measure this effluent’s total strong acid concentration, called “free mineral acidity (FMA).” FMA act as the indicator for the regeneration process.
When the FMA value drops sharply, it explains, the resin bed gets saturated with cations in water, and no more efficient exchange of cations with hydrogen ions. It simply shows the minor release of H+ ions into the water and less generation of strong acid in effluent water. With the low FMA values, you need to regenerate the cation bed to return the resin beds to their hydrogen form. Sulfuric acid is available at an affordable cost; therefore, in most cases, sulfuric acid can be used to regenerate the resin bed. But it would be best if you were keen on improper sulfuric acid consumption to prevent the irreversible fouling (with calcium sulfate) of the cation resin bed.
What can we do to reduce sulfuric acid fouling? You can apply sulfuric acid for high flow rates and use 2% or more minor initial acid concentrations.
Due to the regeneration level, flow rates, sodium leakages can occur, and the FMA level can be slightly lower than the theoretical mineral acidity level. Some installations use Hydrochloric acid for their regeneration process. You can have better clarification on regeneration and the regeneration process by contacting your supplier.
What happens within the anion resin column?
Anion resin column is required to complete the deionization process. Effluent water from the cation resin vessel passes through the anion resin bed. Strong base anion resin beads are in the form of hydroxide. Hydrogen ions in the strong acids in effluent replaced with hydroxide ions release water molecules. Refer to the following illustration; R is the resin bead.
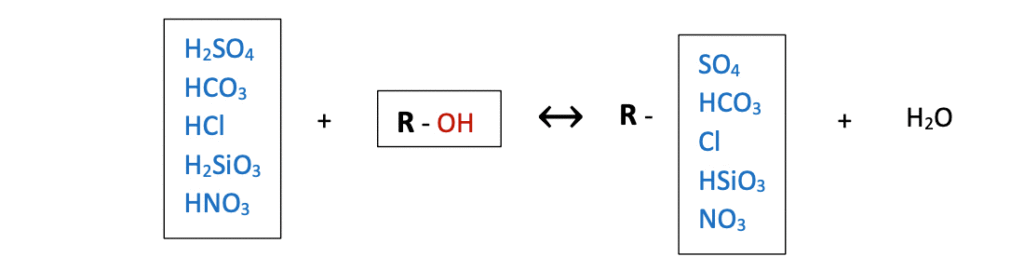
Careful observation of the above indication shows the complete removal of cation and anion ions in feed water. In the real world, ion exchange reactions are equilibrium reactions and can occur some leakages. Sodium leakages are the most common, converted into sodium hydroxide in the anion resin bed. Therefore, two-bed anion-cation DI units produce slightly alkaline effluent water.
Strong base anion (SBA) resins are regenerated with sodium hydroxide solution in the deionization process. SBA resins also remove silica in water. The essential parameters of effluent water are effluent silica level and conductivity. Caustic can result in anions and will be a cause for small leakages of silica. The capacity of leakage depends on the dosage of regenerant and the chemistry of processed water. Silica can be removed from the resin bed with heated sodium hydroxide up to the given temperature by the manufacturer.
What will be the most suitable resin types in the deionization process?
As per the above paragraphs, you know two different cations are used in water deionization. They are cation resins and anion resins. Let’s consider how these resins work during the ion exchanging process.
Cation resins consist of negatively sulfonic groups bonded with hydrogen ions. These resins capture positively charged cations from water and release an equivalent amount of hydrogen ions. The anion resins consist of positively charged ammonium groups and are pre-charged with hydroxide ions. They capture the anions in water and release hydroxide ions into the water. The release of hydrogen ions and hydroxide ions into the water also generates water molecules.
There are two categories of cation resins: strong Acid Cations (SAC) and Weak Acid Cations (WAC). WAC is not made from crosslinked styrene but is commonly made with acrylic crosslinked DVB. Usually, WACs are taken to the partial DI applications or dealkalization. SAC resins are stronger than WAC, and they have a high capacity. H+ form of these SAC resins exchanged all the cations by converting their salts into relevant acids.
Weak base anions (WBA) and strong base anions (SBA) are the two types of DI resins. WBA is not used in the Di process because it adsorbed acid molecules, does not exchange hydroxyl groups, and doesn’t generate water as a byproduct. Also, they cannot reduce CO2 gas (it is present in the cation resin bed with ion-exchange step in low pH) and silica.
According to the above details, the most suitable resin types for the DI process are SAC and the SBA. For the complete deionization or demineralization to produce ultra-pure water in different industries, use mixed-bed resins.
Have you known about mixed bed (MB) resins?
If you have carefully read this article, now you will be able to understand mixed bed resins. An MB is a combination of SAC and SBA in a 2:3 ratio. The resistivity or electrical conductivity is the only way to measure the mixed bed water because it is deficient in dissolved ions. The main point of success of the MB unit is continually working to neutralize the pH values. MB resins can deliver high-quality water with higher capacities to the given quantity of MB resins than the two-bed resin DI system.
The only disadvantage of the MB resins is the highly complex regeneration process. It also needs more equipment.
What will be the physical structure of the resins?
It can be either a gel structure or a macroporous structure. Gel-type resins are cheaply available. Typically, they are transparent and haven’t distinct pores. Its’ mechanism is diffusion.
Macroporous resins are opaque and have distinct, continuous channels. They have increased surface area than gel resins. So these resins have gained good kinetics. Also, they are strong to oxidizing conditions due to the high level of crosslinking.
Conclusion
- Deionization or demineralization produces high-quality water with similar qualities to distilled water.
- Deionization can be done with different methods alone or as a combination of the followings. Ion-exchange, EDI (electro deionization), reverse osmosis, and Nanofiltration. This article has discussed the ion exchanging process.
- There are two types of DI resins; cation resins and anion resins.
- As usual, cation resins exchange their Hydrogen ions with cations in water, and anion resins release OH- into the water by attracting the anions from water.
- The byproduct of the DI process is water.
- Di unit can use these two different resins in two different ways; two-bed resin units and mixed bed resin units.
- Mixed bed resin contains a mix of cation and anion resin in a 2:3 ratio in the same column.
- The most efficient and cost-effective method is MB deionization. But the main dropdown of the system is having a complicated regeneration process with more equipment.

