Welcome to our webpage dedicated to Felite™ Ready-to-use Mixed bed (DI) Resin, a high-quality product that we manufacture to meet the needs of the water purification industry.
Home » Ready-to-Use Mixed Bed(DI) Resin
Home » Ready-to-Use Mixed Bed(DI) Resin
If you’re in need of high-quality demineralized water, Felite™ Ready-to-use Mixed bed (DI) Resin is the ideal solution for you. Our mixed bed resin is specially designed to polish process water to achieve demineralized water quality, typically after passing through a reverse osmosis system. By passing water through our resin at the recommended flow rates, you can achieve almost complete reduction of total dissolved solids.
Our mixed bed resin is specially formulated with a mixture of strong acid cation resin in H+ form and strong base anion resin in OH- form to provide effective and efficient demineralization of water. This allows for the production of high-quality demineralized water with exceptional resistivity.
You can trust Felite™ Ready-to-use Mixed bed (DI) Resin to meet your demineralized water needs with the highest level of quality and reliability.
We offer options for resistivity levels of up to 10MΩ, 15MΩ, or 18MΩ, making it an excellent choice for a wide range of applications that require high-quality demineralized water, such as the pharmaceutical, electronics, Aquarium and power industries.
Mixed bed resin is a type of ion exchange resin used in the demineralization process to produce high-quality, demineralized water. It is unique as it contains both strong acid cation resin in H+ form and strong base anion resin in OH- form within a single unit, enabling the removal of both positively and negatively charged ions from water.
Mixed bed resin comes in two types: gel/gel and gel/macroporous. Gel/gel is the most commonly used type and finds its application in industries requiring deionized water, such as electronics, pharmaceuticals, and aquariums. It is composed of small beads with a consistent gel structure, efficiently removing total dissolved particles from water.

Gel/macroporous type is less commonly used and utilizes macroporous type strong base anion (SBA) resin instead of gel type. It has a high capacity for total dissolved solids removal and is used in the demineralization and decontamination of radioactive effluents.
Mixed bed resin is frequently used in conjunction with other demineralization techniques like reverse osmosis to achieve the highest level of water purity. The positively charged ions are converted to hydrogen ions and negatively charged ions to hydroxide ions as water passes through mixed bed resin, producing high-quality, demineralized water devoid of other contaminants and total dissolved solids and result in pure water with a resistivity level of up to 10MΩ, 15MΩ, or 18MΩ to suit for use in different industries, such as laboratory, power generation, spotless rinsing, aquarium, and electronics manufacturing, etc. Its high capacity to remove TDS(total dissolved solids) makes it a cost-effective and efficient method for producing high-quality, deionized water.
When selecting a mixed bed resin for your application, several factors must be considered to ensure that you choose the right product.

The quality of water required for your application is the first factor to consider. Different applications may have varying water purity requirements, and mixed bed resin can produce water with customized resistivity levels to meet specific needs. Knowing your application’s water quality requirements is crucial before selecting a mixed bed resin.
The type of application is another critical factor. Different industries and applications may require different types of mixed bed resin to achieve optimal performance. For instance, producing ultrapure water for a laboratory application may require a different type of mixed bed resin than producing demineralized water for an aquarium.
The operating conditions of your application also matter when selecting mixed bed resin. For instance, if you operate in a high-temperature environment, a macroporous mixed bed resin that can withstand those conditions may be necessary. Macroporous mixed bed resins have a larger pore size than standard gel structure mixed bed resins, making them more resistant to fouling and better able to withstand high-temperature environments.
Another important factor to consider is whether the mixed bed resin requires regeneration. Mixed bed resin is used as a disposable product in some applications, while in others, it needs to be regenerated. Felite Resin Technology uses black-colored cation component mixed with the standard anion component. This resin type allows for the clear separation of cation and anion resin layers during regeneration. On the other hand, indicator resins use a color change to indicate when the resin needs to be regenerated, providing a more visible indication.
Selecting the right mixed bed resin is crucial for achieving optimal water purity in various applications. If you have any questions or concerns about selecting the right mixed bed resin for your application, don’t hesitate to contact us for expert guidance and support.
In applications where mixed bed resin requires regeneration, it’s important to follow the manufacturer’s guidelines carefully to ensure effective regeneration, which is a crucial process to maintain the quality and effectiveness of the resin bed. The frequency of regeneration depends on the quality of the water being treated and the specific characteristics of the mixed bed resin being used. In general, mixed bed resin should be regenerated once it has reached its exhaustion point, which is determined by monitoring the quality of the water being produced.
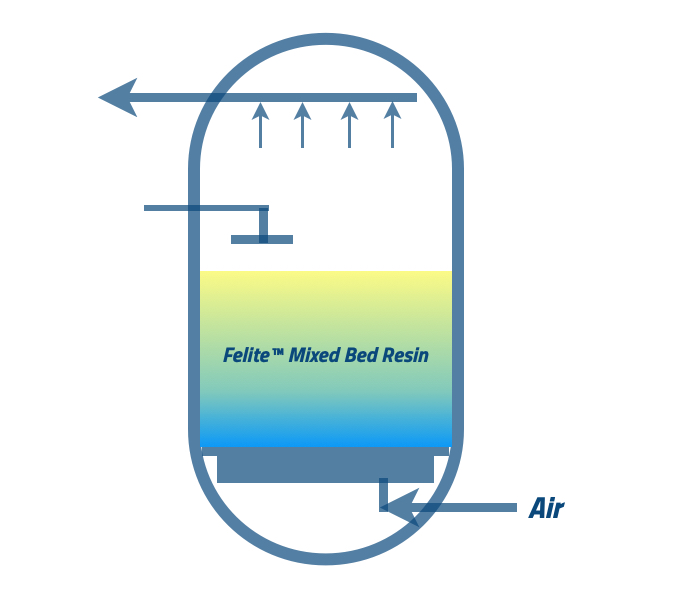
The specific regeneration procedures for mixed bed resin can vary depending on the resin type. In general, the process involves:-
Backwash → flushing the resin bed with a regenerating agent → Mixing → Final Rinse → Disinfection
To get a more detailed explanation of the regeneration steps, please read our blog post《Mixed Bed (DI) Resin Regeneration Process》
Once the mixed bed resin has been regenerated, it should be rinsed and stored properly until it is ready to be used again. Store regenerated resin in a clean, dry container, and ensure that it is properly labeled to avoid any confusion with new resin.
By the best practices for the regeneration of mixed bed resin above, you can ensure that it continues to provide high-quality, demineralized water for your application. If you have any questions or concerns about the regeneration process, don’t hesitate to contact us for expert guidance and support.

Pablo Hensley - Supply Chain Coordinator

Alex Allman - Purchasing Manager
In applications where mixed bed resin requires regeneration, it’s important to follow the manufacturer’s guidelines carefully to ensure effective regeneration, which is a crucial process to maintain the quality and effectiveness of the resin bed. The frequency of regeneration depends on the quality of the water being treated and the specific characteristics of the mixed bed resin being used. In general, mixed bed resin should be regenerated once it has reached its exhaustion point, which is determined by monitoring the quality of the water being produced.
The specific regeneration procedures for mixed bed resin can vary depending on the resin type. In general, the process involves:-
Backwash → flushing the resin bed with a regenerating agent → Mixing → Final Rinse → Disinfection
To get a more detailed explanation of the regeneration steps, please read our blog post《Mixed Bed (DI) Resin Regeneration Process》
Once the mixed bed resin has been regenerated, it should be rinsed and stored properly until it is ready to be used again. Store regenerated resin in a clean, dry container, and ensure that it is properly labeled to avoid any confusion with new resin.
By the best practices for the regeneration of mixed bed resin above, you can ensure that it continues to provide high-quality, demineralized water for your application. If you have any questions or concerns about the regeneration process, don’t hesitate to contact us for expert guidance and support.
Continuous operation of IX resin exhausts with exchange ions gradually decreases the resin efficiency. The regeneration procedure is similar to the IX resin procedures of each resin type.
In the chloride anion dealkalization, anion resin can regenerate with brine solution. It can return the anion resin to its pre-form of chloride. Adding a small amount of caustic soda to the regenerant will increase the alkalinity removal ability.
When we consider the weak acid cation dealkalization, the resin layers can regenerate with an adequately prepared sulfuric acid solution, then with a brine solution. Here, the sodium chloride brine supports the conversion resin to its initial form of Sodium ions. Using sulfuric acid as the regenerant can help the precipitation of calcium sulfate; therefore, maintaining the required acid concentration is a must. HCl acid (with common concentration 5% w/v) also can use for regeneration.