Home » Water Deionization Resin Manufacturer
Home » Water Deionization Resin Manufacturer
Water deionization is a fascinating process that strips water of its mineral ions using ion exchange resins. Think of it as a meticulous purification dance, where unwanted ions in water are replaced with ions that form pure water. It’s crucial in many sectors, from pharmaceuticals to electronics, where water purity is non-negotiable.
Felite™ Resin Technology supplies the best resin types for water deionization applications. According to the selected deionization process, SAC, SBA or mixed bed resin types can be used.
Home » Water Deionization Resin Manufacturer
Felite™ Resin Technology supplies the best resin types for dealkalization application. According to the selected dealkalization process, SAC, WAC, and SBA type-II resin types can be used. The following specially manufactured anion resins can use in dealkalization along with Felite™ strong acid cations in appropriate conditions.



As an industry expert in ion exchange resins, I’ve seen firsthand the transformative power of water deionization. It’s more than just a process; it’s a cornerstone in maintaining the purity standards in many industries. Let me guide you through the essentials of water deionization, blending technical expertise with a sprinkle of my personal insights.

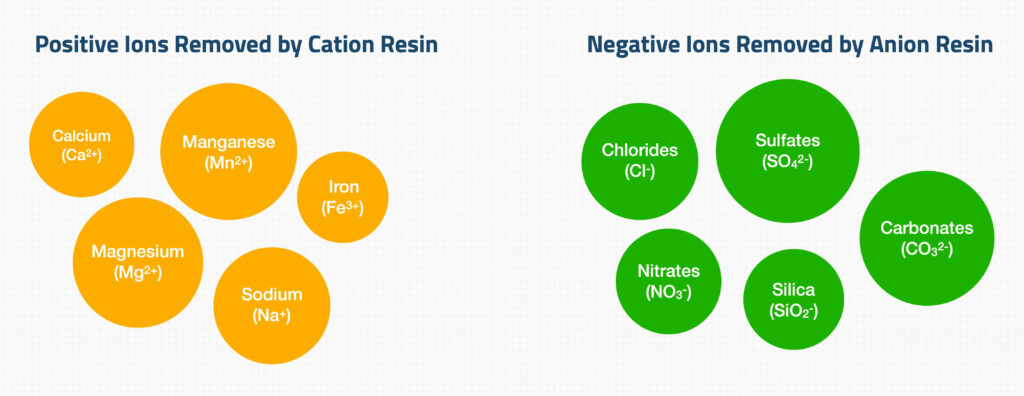
In layman’s terms, water deionization is like a filter on steroids. It uses ion exchange resins to remove mineral ions from water – minerals like calcium, magnesium, and even sodium. These ions are the culprits behind water hardness and conductivity.
Ion exchange resins are the unsung heroes here. They swap the unwanted ions in water for ions that form H2O, leaving you with water in its purest form. It’s a bit like magic, but with science.
Step 1: Pre-treatment
First things first, let’s talk about the 1st step, pre-treatment. It’s like prepping for a marathon – you need to be in top shape before the big event. Pre-treatment involves filtering out sediments and removing chlorine to protect the ion exchange resins. This step is crucial to avoid fouling the resins and to extend their service life. Read about water pre-treatment. Then comes the star of the show, the ion exchange process.
Step 2: Ion exchange process (Separated Bed Deionization)
This process happens in a separated bed(dual-bed) system. Using two separate vessels for anionic and cationic exchange resins, it efficiently strips water of its mineral ions.
In the first cation vessel, cationic impurities are replaced by hydrogen ions from the cation exchange resin. This initial stage effectively removes positive ions from the water. It’s a straightforward swap – two hydrogen ions for every positive ion.
The next step involves the anion exchange vessel. Here, water with a pH concentration of 2 to 2.5, indicative of its high hydrogen ion content, undergoes further purification. The anion resin releases hydroxyl ions, replacing the negative impurities like chloride ions. The result is high-quality deionized water, ready for immediate use in various applications.
While they don’t quite reach the purity levels of mixed-bed deionizers, their considerably high electrical resistivity makes them suitable for many applications. Their separate vessels allow for specific control over the ion exchange process, accommodating distinct flow rates and exchange capacities. This customization is particularly advantageous for certain industrial processes requiring specific water quality parameters.
Step 3: Post-treatment (Polishing by Mixed Bed Deionization, optional)
Post-treatment in water deionization, particularly through mixed bed deionization, is an optional yet crucial step for achieving ultra-pure water. In this stage, a mixed bed system intermixes strong acid cation (SAC) and strong base anion (SBA) resins. This combination allows for continuous and comprehensive ion exchange within the resin bed. Essentially, this setup functions as numerous dual bed units in one, addressing issues like sodium leakage prevalent in separate bed systems. Sodium leakage, where sodium ions bypass the cation resin without being exchanged, is significantly mitigated in a mixed bed system. This is due to the high number of cation/anion exchanges that occur, ensuring the production of high-quality deionized water with minimal impurities.
Separated bed and mixed-bed systems each have their unique applications and advantages in water deionization. In a separated bed system, also known as a dual bed system, deionization occurs in two distinct stages. First, the cation resin removes positively charged ions by exchanging them for hydrogen ions. Then, the water, now containing predominantly hydrogen ions, moves to the anion resin tank, where negatively charged ions like chloride are exchanged for hydroxyl ions, forming water (H2O). However, one of the limitations of dual bed systems is the occurrence of sodium leakage, which can affect water quality.
Mixed bed systems, on the other hand, combine SAC and SBA resins in a single tank, essentially operating as multiple dual bed units in one. This configuration allows for a greater number of ion exchanges, effectively addressing sodium leakage and producing higher quality deionized water compared to separated bed systems. The mixed bed system’s ability to continuously exchange ions within the resin bed results in the production of the highest quality deionized water possible.
Mixed bed resins are like the all-in-one tool in your toolbox. They combine cation and anion exchange resins in one vessel, giving you a one-two punch of purification. It’s particularly effective in the final stages of water purification, where we aim for the highest purity.
Separated bed systems are great for large-scale operations or specific ion removal. But for that extra level of purity, mixed bed resins are your best bet. They’re the secret ingredient in achieving top-notch water quality.
The longevity of mixed bed DI resins depends largely on the quality of the feed water. Think of it as a car’s mileage – the rougher the roads, the more wear and tear on the car. Similarly, tap water with high mineral content will shorten the resin’s lifespan, while treated water is gentler and extends its life.
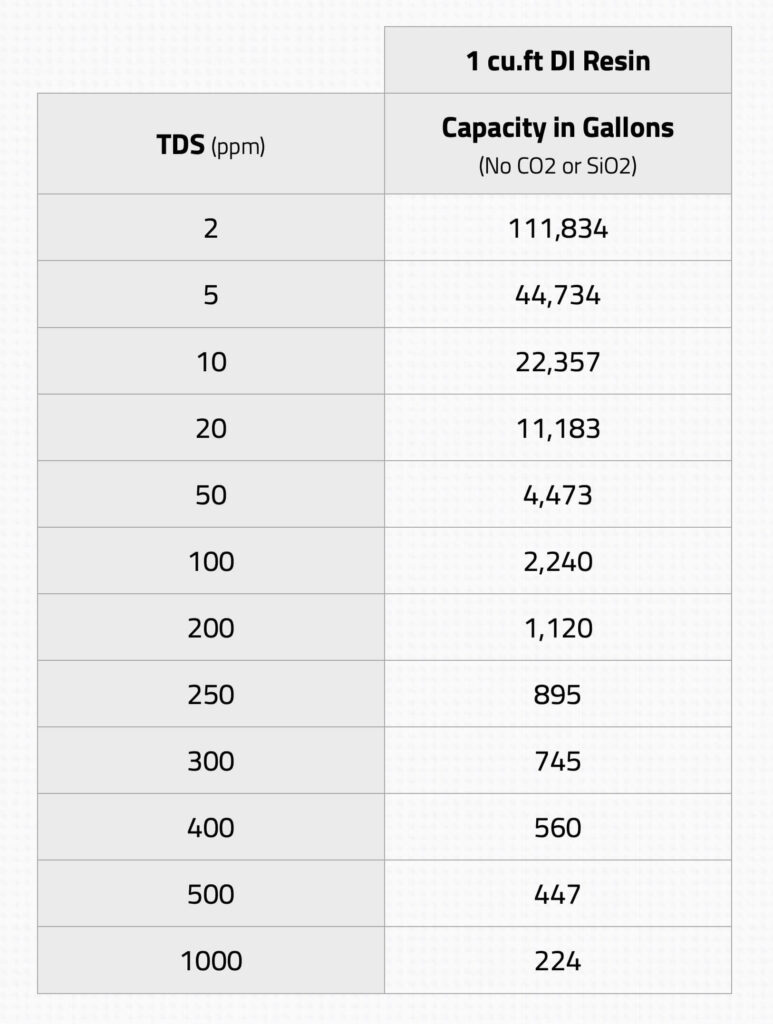
And why is the 40:60 cation to anion ratio so popular? It’s all about balance. This ratio is found to be optimal for a wide range of water qualities, providing effective ion removal while maintaining the resin’s longevity and minimizing regeneration frequency. It’s the golden ratio of the water deionization world.
Mixed bed resins are like the all-in-one tool in your toolbox. They combine cation and anion exchange resins in one vessel, giving you a one-two punch of purification. It’s particularly effective in the final stages of water purification, where we aim for the highest purity.
Separated bed systems are great for large-scale operations or specific ion removal. But for that extra level of purity, mixed bed resins are your best bet. They’re the secret ingredient in achieving top-notch water quality.
In applications where mixed bed resin requires regeneration, it’s important to follow the manufacturer’s guidelines carefully to ensure effective regeneration, which is a crucial process to maintain the quality and effectiveness of the resin bed. The frequency of regeneration depends on the quality of the water being treated and the specific characteristics of the mixed bed resin being used. In general, mixed bed resin should be regenerated once it has reached its exhaustion point, which is determined by monitoring the quality of the water being produced.
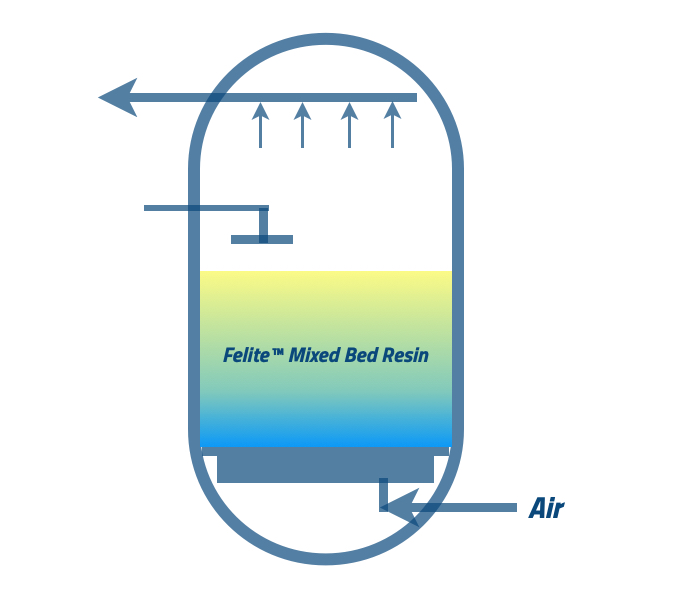
The specific regeneration procedures for mixed bed resin can vary depending on the resin type. In general, the process involves:-
Backwash → flushing the resin bed with a regenerating agent → Mixing → Final Rinse → Disinfection
To get a more detailed explanation of the regeneration steps, please read our blog post《Mixed Bed (DI) Resin Regeneration Process》
Once the mixed bed resin has been regenerated, it should be rinsed and stored properly until it is ready to be used again. Store regenerated resin in a clean, dry container, and ensure that it is properly labeled to avoid any confusion with new resin.
By the best practices for the regeneration of mixed bed resin above, you can ensure that it continues to provide high-quality, demineralized water for your application. If you have any questions or concerns about the regeneration process, don’t hesitate to contact us for expert guidance and support.
Water quality expresses the chemical, physical, and biological characteristics levels concerning its targeted purposes, such as drinking, processing, or swimming.
Do you know what alkalinity is in water? Yes. It is the acid neutralizing capacity of water. Also, it is a measure of the aggregate property of water. With the known chemical composition of your water sample, alkalinity can be interpreted in terms of specific substances. Also, alkalinity is a chemical parameter that expresses the acid and base neutralizing capacity of the water body or buffering capacity of water and maintaining its stable pH level. Alkalinity presents due to hydroxide ions, carbonate ions, and bicarbonate ions in water. In most cases, bicarbonates with carbon dioxide create bulk alkalinity in natural water. Alkalinity in naturally occurring water is a function of pH water.
Alkalinity in water combined with water hardness will be a critical contaminant in industrial water-consuming processes. High water consumers have many issues in commercial sectors due to alkalinity together with hardness. It should be removed and under control to scaling & corrosion problems to reduce the operational and maintenance cost. Scaling potential is more significant in industrial heat-changing equipment due to higher heat transfer rates and high operating temperatures. Water treatment specialists introduce new technologies for water purification.
Alkalinity plays a significant role in boiler operations.
Removing alkaline ions from water is called “dealkalization.” Some amine compounds can protect the condensate lines from corrosion, but it is not a cost-effective method. More amine is required with more alkaline feed water. The boiler operating process requires hardness removal and reduction of alkalinity with no removal of other solids. In the water treatment industry, process engineers prioritize membrane technology and ion exchange technology for dealkalization. In most cases softening doesn’t remove alkalinity, but demineralization does at a high cost.
Yeah, we can use the IX process in different engineering designs for dealkalizers. Various chemical processing applications use ion exchange (IX) and can be identified into three main categories.
In the first case, IX resins remove simple inorganic and organic electrolytes. There are many resin types according to the operating conditions. The main four categories are strong acid cations (SAC), weak acid cations (WAC), strong base anions (SBA), and weak base anions (WBA) resin types. Also, these four main types have gel type and macro-porous type, as well as styrene, acrylic, or formophenolic types. But, resin manufacturers develop unique resin chemical structures for high selectivity of ions. Combining two different functional groups on the same resin increases the selectivity and the exchange kinetics. The second case is frequently used for applications on organic compounds. Adsorption involves as the main mechanism. Unique resin chelates are used to remove the metal complexes.
Water treatment specialists introduced many technologies for the dealkalization process, and this article will focus on ion exchange technology. Felite™ Resin Technology has introduced different resin types for dealkalization and manufacturing specialized four resins as mentioned above.
Dealkalization is one procedure that uses IX resins to purify the water. We will further discuss FeliteTM resin types which suit the dealkalization process.
We’d love to answer any of your technical questions !
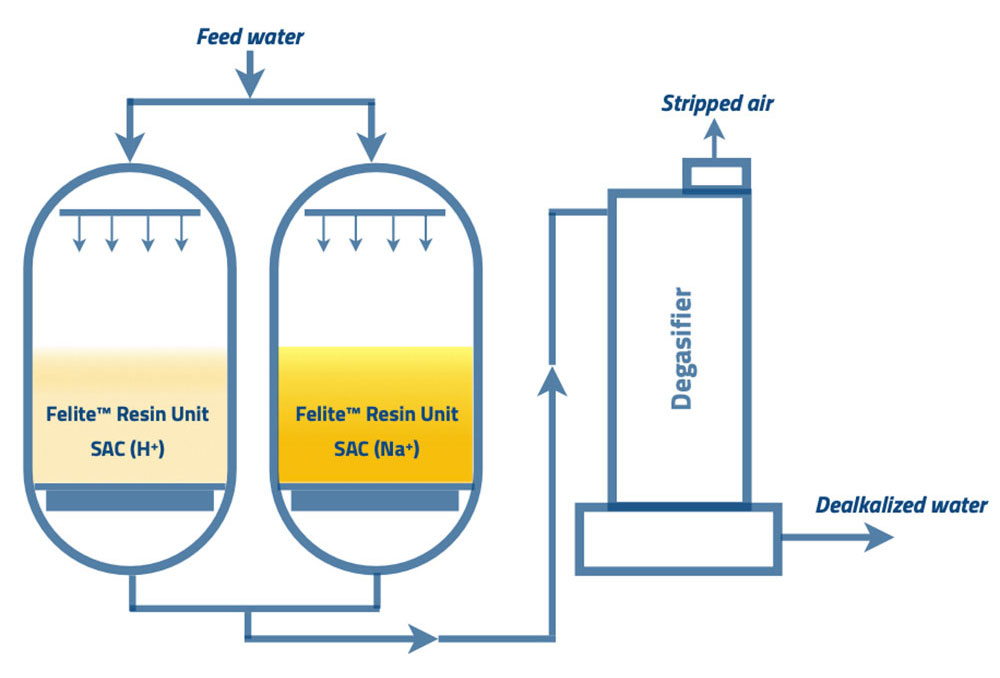
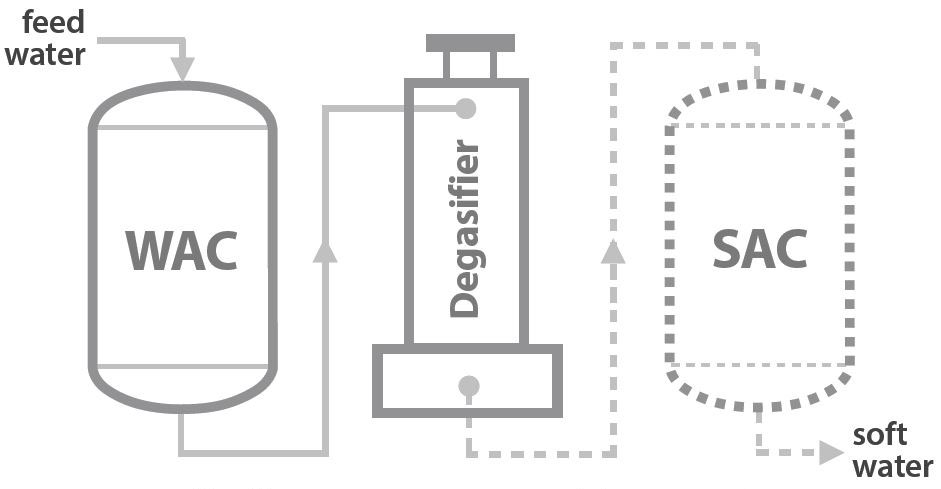
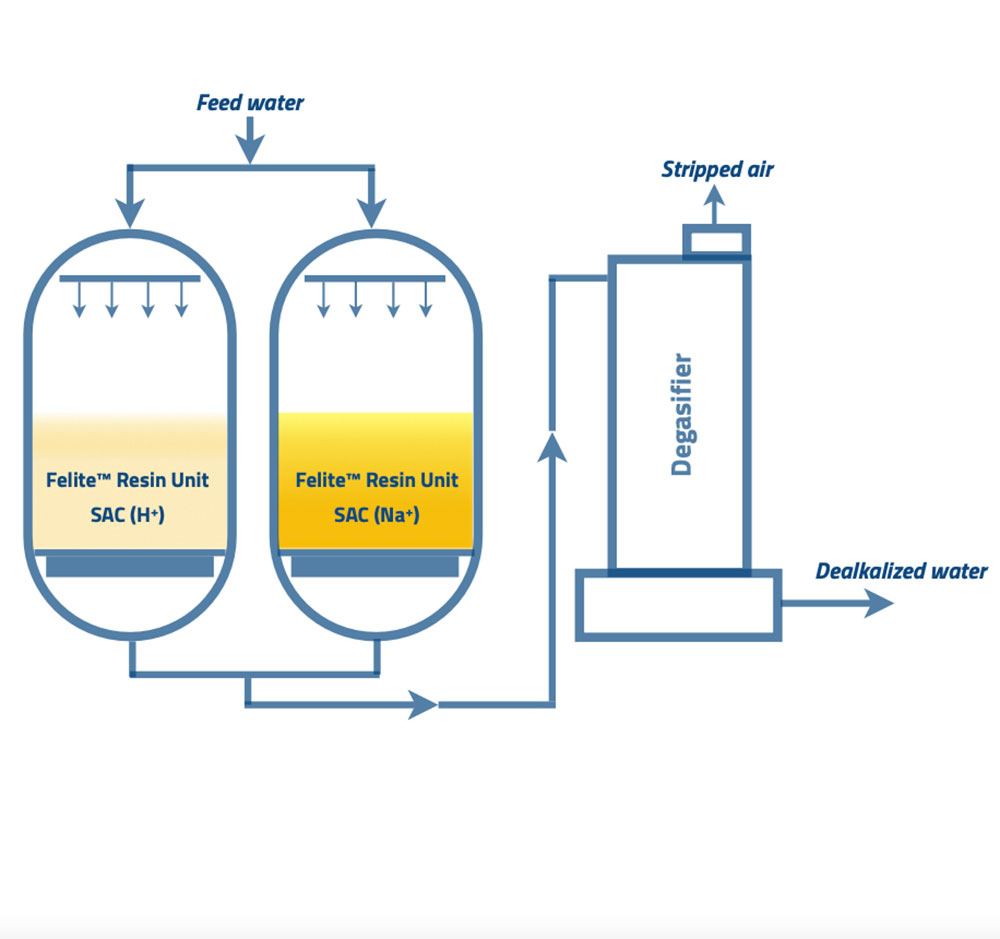
The process flow diagram shows the main summary of the split stream dealkalization process. Two strong acid resin beds are operated in two different forms in parallel; one column has SAC resins in Na+ form. The other vessel has SAC resins in H+ form. The resin bed in the sodium form act as a softener unit. The additional resin bed in hydrogen form operates as a demineralizing unit.
The influent feed water is split into two streams. One stream enters the cation resin in the sodium form column (A column), and the remainder enters the resin in the hydrogen form column (B column). The outlet water from column “A” contains 100% of influent alkalinity, and water from column “B” contains zero alkalinity and free mineral acidity (FMA). In the next step, these two streams blend. FMA in the hydrogen cation resin effluent converts sodium carbonate and bicarbonate alkalinity in the sodium cation resins effluent to carbonic acid. Refer to the following reactions for further understanding.
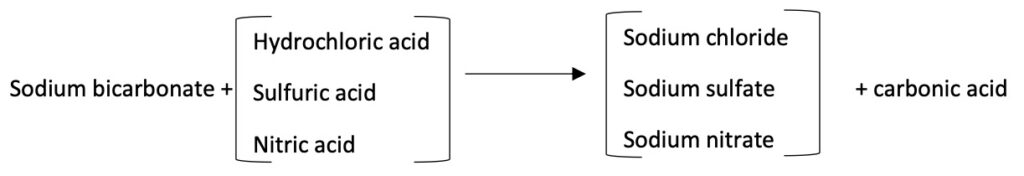
Carbonic acid (H2CO3) dissociation in water produces predominant species as follows.
H2CO3 ←→ H2O + CO2
Therefore, engineers set up the blended water to enter the degassifier. The countercurrent air stream will strip out carbon dioxide gas from the combined water. The final water alkalinity can be controlled by managing the percentages of each mixed water flow. The main advantage of the split stream dealkalization method is maintaining the desired alkalinity level of the final effluent water.
Strong acid cation resins have a higher operating capacity, reducing the required resin volume. FeliteTM introduces strong acid cation resins, a macro-porous type for effective treatment in dealkalizers. Using sulfuric acid as the regenerant of SAC resins is hazardous. You might be able to allocate extra capital costs and operational costs for the degassifier.
In summary, there are positive points in the split stream method, and they are;
Also, there are some downsides to this method;
We’d love to answer any of your technical questions !
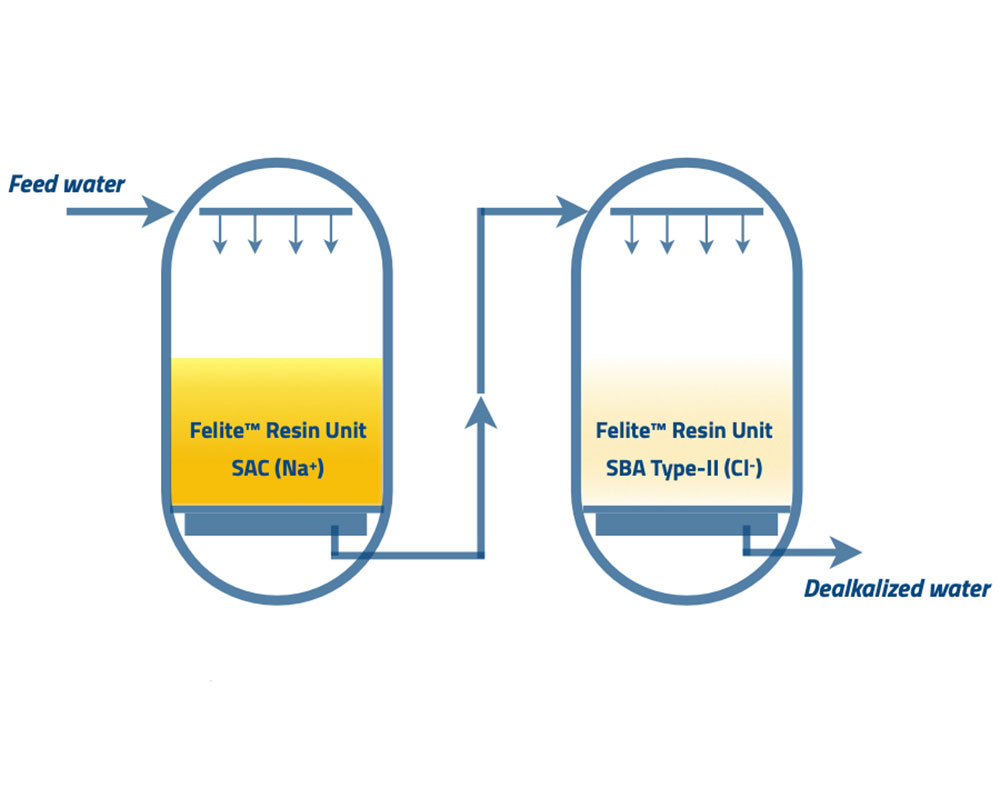
The chloride anion dealkalization process is the most prevalent in lite industrial applications commonly used for low-pressure boiler operations. A softener unit is activated before an anion unit consisting of the strong base anion (SBA), type-II resins. These resins are present in chloride form.
The following reactions occur at the softener unit to remove hardness and reduce the fouling of SBA resins. Ca2+ and Mg2+ ions are replaced by the monovalent sodium ions attached to the SAC resins in the softener unit. Then sodium ions are released into the water.
Softened water enters the chloride anion dealkalizers with type-II SBA resins to reduce the alkalinity and the sulfates. Follow the ion exchange reactions shown below.
The chloride ions coming through the softener unit do not change. The salt splitting de-alkalization process has 90% efficiency in reducing the alkalinity in water without lowering the total solids. Type II SBA resins have a higher affinity to sulfate ions than alkaline ions, minimizing the resin efficiency and affecting the alkalinity leakages. The low ratio of sulfate ions to the total anions or high ratio of alkalinity to the total anions may increase the resin efficiency and decrease the alkalinity leakages.
The rapid increase of treated water’s alkalinity indicates the resin’s exhaustion and shows the regeneration requirement. Softener resins and the strong base anion type-II resin can use the brine solution to regenerate the resins as per the same procedure of softener resin regeneration. Using brine as the regenerant is safer here than in the process of the split stream.
Another point in regeneration, if caustic is used as a brine regenerant in the dealkalizing column, free CO2 in water is converted into bicarbonates or carbonates by exchanging with chloride ions. Reducing alkalinity decreases the carbon dioxide (CO2) in boiler condensate resulting in a low concentration of amine. Due to the stable TDS amount in alkaline water, there is no increase in the boiler’s concentration cycles. There can be slight increases in conductivity in boiler feed water.
Great attention is required for magnesium leakages from the softener. If magnesium hardness is higher than 1ppm, there is a chance to produce HCl after anion resin column in the condition of zero alkalinity.
MgCl2 + H2O → Mg(OH)2 + HCl
Excess softener capacity can be converted to a delikalizer, which will be a good advantage of this process. There is no initial capital cost for installing the degassifier and no operational cost.
Salt splitting de-alkalizer performs few disadvantages.
We’d love to answer any of your technical questions !
Weak acid cation dealkalization treats raw water with both hardness and alkalinity. The influent water, either with a similar hardness level to the alkalinity level or a lesser hardness level to the alkalinity level, is the best feeding water to the WAC dealkalizers. It is a cost-effective and highly efficient system for the above condition. In the case of higher hardness than the alkalinity level, dealkalized water remains some hardness. Therefore, a softener unit must follow the WAC dealkalizers to remove the remaining hardness.
The weak acid cations are in the form of H+. WAC resins act similar to SAC resins but exchange only the cations associated with the alkalinity. The effluent water from the WAC dealkalizers enters the degassifier due to the formation of carbon dioxide by dissociation of carbonic acid. The following equation is similar to the Mg2+ and Na+ cations too.
Ca(HCO3)2 +2R-H → 2R-Ca + 2H2CO3 (R – represents the resin)
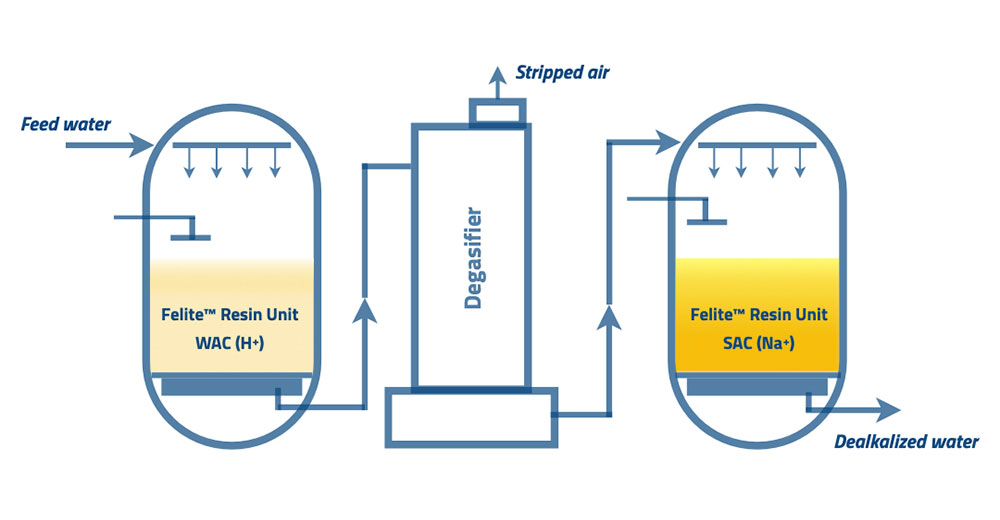
The generated carbonic acid dissociates into water and carbon dioxide. Carbon dioxide will be stripped through a countercurrent air stream within the decarbonator. Also, it removes the TDS. The softener unit will remove the remaining permanent hardness during the effluent process through the degassifier or decarbonator. Adding a small amount of caustic to maintain the desired pH level in final effluent water is better.
When the resins near exhaustion, the effluent alkalinity level is higher than influent alkalinity. At that time, the regeneration process needs to proceed. WAC resins can regenerate with sulfuric acid and the softener resins with the brine solution.
WAC dealkalizers have high operating capacities. WAC resins are capable of removing bulk hardness during dealkalization. Therefore softener has minimized its capacity. The high operating efficiency of WAC resins reduces the amount of acidic regenerant waste.
Weak acid cation dealkalization treats raw water with both hardness and alkalinity. The influent water, either with a similar hardness level to the alkalinity level or a lesser hardness level to the alkalinity level, is the best feeding water to the WAC dealkalizers. It is a cost-effective and highly efficient system for the above condition. In the case of higher hardness than the alkalinity level, dealkalized water remains some hardness. Therefore, a softener unit must follow the WAC dealkalizers to remove the remaining hardness.
The weak acid cations are in the form of H+. WAC resins act similar to SAC resins but exchange only the cations associated with the alkalinity. The effluent water from the WAC dealkalizers enters the degassifier due to the formation of carbon dioxide by dissociation of carbonic acid. The following equation is similar to the Mg2+ and Na+ cations too.
Ca(HCO3)2 +2R-H → 2R-Ca + 2H2CO3 (R – represents the resin)
The generated carbonic acid dissociates into water and carbon dioxide. Carbon dioxide will be stripped through a countercurrent air stream within the decarbonator. Also, it removes the TDS. The softener unit will remove the remaining permanent hardness during the effluent process through the degassifier or decarbonator. Adding a small amount of caustic to maintain the desired pH level in final effluent water is better.

When the resins near exhaustion, the effluent alkalinity level is higher than influent alkalinity. At that time, the regeneration process needs to proceed. WAC resins can regenerate with sulfuric acid and the softener resins with the brine solution.
WAC dealkalizers have high operating capacities. WAC resins are capable of removing bulk hardness during dealkalization. Therefore softener has minimized its capacity. The high operating efficiency of WAC resins reduces the amount of acidic regenerant waste.
We’d love to answer any of your technical questions !

Pablo Hensley - Supply Chain Coordinator

Alex Allman - Purchasing Manager
Continuous operation of IX resin exhausts with exchange ions gradually decreases the resin efficiency. The regeneration procedure is similar to the IX resin procedures of each resin type.
In the chloride anion dealkalization, anion resin can regenerate with brine solution. It can return the anion resin to its pre-form of chloride. Adding a small amount of caustic soda to the regenerant will increase the alkalinity removal ability.
When we consider the weak acid cation dealkalization, the resin layers can regenerate with an adequately prepared sulfuric acid solution, then with a brine solution. Here, the sodium chloride brine supports the conversion resin to its initial form of Sodium ions. Using sulfuric acid as the regenerant can help the precipitation of calcium sulfate; therefore, maintaining the required acid concentration is a must. HCl acid (with common concentration 5% w/v) also can use for regeneration.
With five decades of experience, Felite Resin Technology is well versed in this field. In other hand, you are dealing with the master. You can trust us with your needs and expectations.
In the past five decades, we’ve developed several ion exchange resin models as per different customers and applications required, from standard mesh to fine mesh; from industrial grade to potable water grade.

Want to know how we make doing business easier? Let’s talk.